Abstract
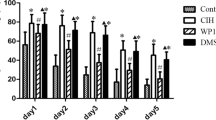
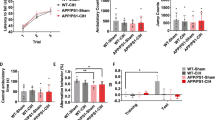
Obstructive sleep apnea–hypopnea syndrome (OSAHS) is typically characterized by chronic intermittent hypoxia (CIH), associated with cognitive dysfunction in children. Calcium-sensing receptor (CaSR) mediates the apoptosis of hippocampal neurons in various diseases. However, the effect of CaSR on OSAHS remains elusive. In the present study, we investigated the role of CaSR in CIH-induced memory dysfunction and underlying mechanisms on regulation of PKC-ERK1/2 signaling pathway in vivo and in vitro. CIH exposures for 4 weeks in mice, modeling OSAHS, contributed to cognitive dysfunction. CIH accelerated apoptosis of hippocampal neurons and resulted in the synaptic plasticity deficit via downregulated synaptophysin (Syn) protein level. The mice were intraperitoneally injected with CaSR inhibitor (NPS2143) 30 min before CIH exposure and the results demonstrated CaSR inhibitor alleviated the apoptosis and synaptic plasticity deficit in the hippocampus of CIH mice. We established intermittent hypoxia PC12 cell model and found that the activation of CaSR accelerated CIH-induced PC12 apoptosis and synaptic plasticity deficit by upregulated p-ERK1/2 and PKC. Overall, our findings revealed that CaSR held a critical function on CIH-induced cognitive dysfunction in mice by accelerating hippocampal neuronal apoptosis and reducing synaptic plasticity via augmenting CaSR-PKC-ERK1/2 pathway; otherwise, inhibition of CaSR alleviated CIH-induced cognitive dysfunction.









Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Change history
23 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12035-023-03323-w
References
Marcus CL, Brooks LJ, Draper KA et al (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130:576–584. https://doi.org/10.1542/peds.2012-1671
Veasey SC, Rosen IM (2019) Obstructive sleep apnea in adults. N Engl J Med 380:1442–1449. https://doi.org/10.1056/NEJMcp1816152
Cai X-H, Li X-C, Hu Q-Q et al (2013) Multiple system morbidities associated with children with snore symptom. Pediatr Pulmonol 48:381–389. https://doi.org/10.1002/ppul.22653
Lo Bue A, Salvaggio A, Insalaco G (2020) Obstructive sleep apnea in developmental age. A narrative review. Eur J Pediatr 179:357–365. https://doi.org/10.1007/s00431-019-03557-8
Li X-C, Hong F-F, Tu Y-J et al (2022) Blockade of adenosine A2A receptor alleviates cognitive dysfunction after chronic exposure to intermittent hypoxia in mice. Exp Neurol 350:113929. https://doi.org/10.1016/j.expneurol.2021.113929
Nair D, Ramesh V, Gozal D (2018) Cognitive deficits are attenuated in neuroglobin overexpressing mice exposed to a model of obstructive sleep apnea. Front Neurol 9:426. https://doi.org/10.3389/fneur.2018.00426
Díaz-Soto G, Rocher A, García-Rodríguez C et al (2016) The calcium-sensing receptor in health and disease. Int Rev Cell Mol Biol 327:321–369. https://doi.org/10.1016/bs.ircmb.2016.05.004
Giudice ML, Mihalik B, Dinnyés A, Kobolák J (2019) The nervous system relevance of the calcium sensing receptor in health and disease. Mol Basel Switz 24:E2546. https://doi.org/10.3390/molecules24142546
Noh JS, Pak H-J, Shin Y-J et al (2015) Differential expression of the calcium-sensing receptor in the ischemic and border zones after transient focal cerebral ischemia in rats. J Chem Neuroanat 66–67:40–51. https://doi.org/10.1016/j.jchemneu.2015.05.001
Paquot F, Huart J, Defraigne J-O et al (2017) Implications of the calcium-sensing receptor in ischemia/reperfusion. Acta Cardiol 72:125–131. https://doi.org/10.1080/00015385.2017.1291136
Babiec WE, O’Dell TJ (2018) Novel Ca2+-dependent mechanisms regulate spontaneous release at excitatory synapses onto CA1 pyramidal cells. J Neurophysiol 119:597–607. https://doi.org/10.1152/jn.00628.2017
Guo J, Li H-Z, Wang L-C et al (2012) Increased expression of calcium-sensing receptors in atherosclerosis confers hypersensitivity to acute myocardial infarction in rats. Mol Cell Biochem 366:345–354. https://doi.org/10.1007/s11010-012-1312-0
Vizard TN, Newton M, Howard L et al (2015) ERK signaling mediates CaSR-promoted axon growth. Neurosci Lett 603:77–83. https://doi.org/10.1016/j.neulet.2015.07.019
Arias-Cavieres A, Khuu MA, Nwakudu CU et al (2020) A HIF1a-dependent pro-oxidant state disrupts synaptic plasticity and impairs spatial memory in response to intermittent hypoxia. eNeuro 7:ENEURO.0024-20.2020. https://doi.org/10.1523/ENEURO.0024-20.2020
Goussakov I, Synowiec S, Aksenov DP, Drobyshevsky A (2021) Occlusion of activity dependent synaptic plasticity by late hypoxic long term potentiation after neonatal intermittent hypoxia. Exp Neurol 337:113575. https://doi.org/10.1016/j.expneurol.2020.113575
Conigrave AD, Ward DT (2013) Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 27:315–331. https://doi.org/10.1016/j.beem.2013.05.010
Li X, Yao L, Liang Q et al (2018) Propofol protects hippocampal neurons from hypoxia-reoxygenation injury by decreasing calcineurin-induced calcium overload and activating YAP signaling. Oxid Med Cell Longev 2018:1725191. https://doi.org/10.1155/2018/1725191
Huang A, Binmahfouz L, Hancock DP et al (2021) Calcium-sensing receptors control CYP27B1-luciferase expression: transcriptional and posttranscriptional mechanisms. J Endocr Soc 5:bvab057. https://doi.org/10.1210/jendso/bvab057
Yamada K, Yoshida K (2022) Multiple subcellular localizations and functions of protein kinase Cδ in liver cancer. World J Gastroenterol 28:188–198. https://doi.org/10.3748/wjg.v28.i2.188
Li J, Qu Y, Zu P et al (2006) Increased isoform-specific membrane translocation of conventional and novel protein kinase C in human neuroblastoma SH-SY5Y cells following prolonged hypoxia. Brain Res 1093:25–32. https://doi.org/10.1016/j.brainres.2006.03.110
Lovicu FJ, McAvoy JW (2001) FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Dev Camb Engl 128:5075–5084
Shen C, Cao K, Cui S et al (2020) SiNiSan ameliorates depression-like behavior in rats by enhancing synaptic plasticity via the CaSR-PKC-ERK signaling pathway. Biomed Pharmacother Biomed Pharmacother 124:109787. https://doi.org/10.1016/j.biopha.2019.109787
Wang P, Wang L, Wang S et al (2015) Effects of calcium-sensing receptors on apoptosis in rat hippocampus during hypoxia/reoxygenation through the ERK1/2 pathway. Int J Clin Exp Pathol 8:10808–10815
Du S-J, Zhang Y, Zhao Y-M et al (2021) Astragaloside IV attenuates cerebral ischemia-reperfusion injury in rats through the inhibition of calcium-sensing receptor-mediated apoptosis. Int J Mol Med 47:302–314. https://doi.org/10.3892/ijmm.2020.4777
Wang C, Jia Q, Sun C, Jing C (2020) Calcium sensing receptor contribute to early brain injury through the CaMKII/NLRP3 pathway after subarachnoid hemorrhage in mice. Biochem Biophys Res Commun 530:651–657. https://doi.org/10.1016/j.bbrc.2020.07.081
Zhang Y, Cao H, Qiu X et al (2020) Neuroprotective effects of adenosine A1 receptor signaling on cognitive impairment induced by chronic intermittent hypoxia in mice. Front Cell Neurosci 14:202. https://doi.org/10.3389/fncel.2020.00202
Singh BL, Chen L, Cai H et al (2019) Activation of adenosine A2a receptor accelerates and A2a receptor antagonist reduces intermittent hypoxia induced PC12 cell injury via PKC-KATP pathway. Brain Res Bull 150:118–126. https://doi.org/10.1016/j.brainresbull.2019.05.015
Kuida K (2000) Caspase-9. Int J Biochem Cell Biol 32:121–124. https://doi.org/10.1016/s1357-2725(99)00024-2
Almawi WY, Melemedjian OK, Jaoude MMA (2004) On the link between Bcl-2 family proteins and glucocorticoid-induced apoptosis. J Leukoc Biol 76:7–14. https://doi.org/10.1189/jlb.0903450
Zhang W, Zhu T, Chen L et al (2020) MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am J Physiol Heart Circ Physiol 318:H59–H71. https://doi.org/10.1152/ajpheart.00308.2019
Liu J, Liu D, Zhang X et al (1979) (2021) NELL2 modulates cell proliferation and apoptosis via ERK pathway in the development of benign prostatic hyperplasia. Clin Sci Lond Engl 135:1591–1608. https://doi.org/10.1042/CS20210476
Huang Q, Wang P, Liu H et al (2022) Inhibition of ERK1/2 regulates cognitive function by decreasing expression levels of PSD-95 in the hippocampus of CIH rats. Eur J Neurosci 55:1471–1482. https://doi.org/10.1111/ejn.15635
Greenberg HZE, Jahan KS, Shi J et al (2016) The calcilytics Calhex-231 and NPS 2143 and the calcimimetic Calindol reduce vascular reactivity via inhibition of voltage-gated Ca2+ channels. Eur J Pharmacol 791:659–668. https://doi.org/10.1016/j.ejphar.2016.10.008
Ong Q, Guo S, Zhang K, Cui B (2015) U0126 protects cells against oxidative stress independent of its function as a MEK inhibitor. ACS Chem Neurosci 6:130–137. https://doi.org/10.1021/cn500288n
Zheng H, Su Y, Zhu C et al (2021) An addition of U0126 protecting heart grafts from prolonged cold ischemia-reperfusion injury in heart transplantation: a new preservation strategy. Transplantation 105:308–317. https://doi.org/10.1097/TP.0000000000003402
Mei H-F, Poonit N, Zhang Y-C et al (2018) Activating adenosine A1 receptor accelerates PC12 cell injury via ADORA1/PKC/KATP pathway after intermittent hypoxia exposure. Mol Cell Biochem 446:161–170. https://doi.org/10.1007/s11010-018-3283-2
Glikmann-Johnston Y, Fink KD, Deng P et al (2019) Spatial memory in Huntington’s disease: a comparative review of human and animal data. Neurosci Biobehav Rev 98:194–207. https://doi.org/10.1016/j.neubiorev.2019.01.015
Gong L-J, Wang X-Y, Gu W-Y, Wu X (2020) Pinocembrin ameliorates intermittent hypoxia-induced neuroinflammation through BNIP3-dependent mitophagy in a murine model of sleep apnea. J Neuroinflammation 17:337. https://doi.org/10.1186/s12974-020-02014-w
Snyder B, Duong P, Trieu J, Cunningham RL (2018) Androgens modulate chronic intermittent hypoxia effects on brain and behavior. Horm Behav 106:62–73. https://doi.org/10.1016/j.yhbeh.2018.09.005
Canessa N, Castronovo V, Cappa SF et al (2011) Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 183:1419–1426. https://doi.org/10.1164/rccm.201005-0693OC
Cai X-H, Li X-C, Jin S-W et al (2014) Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp Neurol 257:148–156. https://doi.org/10.1016/j.expneurol.2014.04.029
Gozal E, Gozal D, Pierce WM et al (2002) Proteomic analysis of CA1 and CA3 regions of rat hippocampus and differential susceptibility to intermittent hypoxia. J Neurochem 83:331–345. https://doi.org/10.1046/j.1471-4159.2002.01134.x
Zhu J, Tang S, Zhao D et al (2021) Orexin A improves the cognitive impairment induced by chronic intermittent hypoxia in mice. Brain Res Bull 173:203–210. https://doi.org/10.1016/j.brainresbull.2021.05.022
Yuan X, Guo X, Deng Y et al (2015) Chronic intermittent hypoxia-induced neuronal apoptosis in the hippocampus is attenuated by telmisartan through suppression of iNOS/NO and inhibition of lipid peroxidation and inflammatory responses. Brain Res 1596:48–57. https://doi.org/10.1016/j.brainres.2014.11.035
Gilsoul M, Grisar T, Delgado-Escueta AV et al (2019) Subtle brain developmental abnormalities in the pathogenesis of juvenile myoclonic epilepsy. Front Cell Neurosci 13:433. https://doi.org/10.3389/fncel.2019.00433
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 73:2424–2428. https://doi.org/10.1073/pnas.73.7.2424
Hu Q, Huang L, Zhao C et al (2019) Ca2+-PKCα-ERK1/2 signaling pathway is involved in the suppressive effect of propofol on proliferation of neural stem cells from the neonatal rat hippocampus. Brain Res Bull 149:148–155. https://doi.org/10.1016/j.brainresbull.2019.04.005
Wang Y, Zhang J, Huang Z-H et al (2017) Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis 8:e2876. https://doi.org/10.1038/cddis.2017.265
Mohamed SK, Ahmed AAE, Elmorsy EM, Nofal S (2019) ERK activation by zeranol has neuroprotective effect in cerebral ischemia reperfusion. Life Sci 227:137–144. https://doi.org/10.1016/j.lfs.2019.04.035
Li Y, Yu M, Zhao B et al (2018) Clonidine preconditioning improved cerebral ischemia-induced learning and memory deficits in rats via ERK1/2-CREB/ NF-κB-NR2B pathway. Eur J Pharmacol 818:167–173. https://doi.org/10.1016/j.ejphar.2017.10.041
Allen KD, Gourov AV, Harte C et al (2014) Nucleolar integrity is required for the maintenance of long-term synaptic plasticity. PLoS ONE 9:e104364. https://doi.org/10.1371/journal.pone.0104364
Sim KH, Lee YJ (2022) Perfluorohexane sulfonate induces memory impairment and downregulation of neuroproteins via NMDA receptor-mediated PKC-ERK/AMPK signaling pathway. Chemosphere 288:132503. https://doi.org/10.1016/j.chemosphere.2021.132503
Lee SH, Kwon S-C, Ok S-H et al (2019) Levobupivacaine-induced vasoconstriction involves caldesmon phosphorylation mediated by tyrosine kinase-induced ERK phosphorylation. Eur J Pharmacol 842:167–176. https://doi.org/10.1016/j.ejphar.2018.10.055
Schönwasser DC, Marais RM, Marshall CJ, Parker PJ (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18:790–798. https://doi.org/10.1128/MCB.18.2.790
Li Z, Agrawal V, Ramratnam M et al (2019) Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury. Cardiovasc Res 115:1646–1658. https://doi.org/10.1093/cvr/cvz037
Acknowledgements
We sincerely thank the staff of the Department of Science and Research Center at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University for their constant and unselfish help.
Funding
This work was supported by the Natural Science Foundation of China (81870073), Basic Public Welfare Projects of Zhejiang Province (Q23H010008), Medical Science and Technology Project of Zhejiang Province (2021KY209), and Science and Technology Plan Project of Wenzhou, China (Y20210009).
Author information
Authors and Affiliations
Contributions
Xiucui Li, Wei Wang, and Xiaohong Cai participated in the research design. Huiya Ying, Zijing Yang, Yuanai Li, and Cancan You conducted the experiments and synthesized the data. Huiya Ying, Zilong Zhang, Xiucui Li, and Wei Wang contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animals used in the experiment were cared for in accordance with the ethical guidelines on animal experimentation of Laboratory Animals of China National Institutes of Health.
Consent to Participate
This study did not include the human subjects.
Consent for Publication
This study did not include the human subjects.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ying, H., Zhang, Z., Wang, W. et al. Inhibition of Calcium-Sensing Receptor Alleviates Chronic Intermittent Hypoxia-Induced Cognitive Dysfunction via CaSR-PKC-ERK1/2 Pathway. Mol Neurobiol 60, 2099–2115 (2023). https://doi.org/10.1007/s12035-022-03189-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03189-4