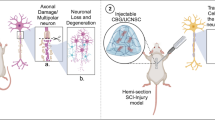
Abstract
Neuroinflammation plays a critical role in the neurological recovery of spinal cord injury (SCI). Adenosine can modulate neuroinflammation, whose uptake is mediated by nucleoside transporters. This study aimed to investigate the roles of equilibrative nucleoside transporter 1 (Ent1) in the inflammatory responses and functional recovery of SCI. Spinal cord contusion at the T10 dorsal portion was induced in mice to cause partial paralysis of the hindlimbs. Genetic deletion and pharmacological inhibition of Ent1 were used to evaluate the role of Ent1 in SCI. The outcomes were evaluated in terms of the Basso Mouse Scale (BMS), gait analysis, astrogliosis, microgliosis, and cytokine levels on day 14 post-injury. As a result, Ent1 deletion reduced neuroinflammation and improved the BMS score (4.88 ± 0.35 in Ent1−/− vs. 3.78 ± 1.09 in Ent1+/+) and stride length (3.74 ± 0.48 cm in Ent1−/− vs. 2.82 ± 0.78 cm in Ent1+/+) of mice with SCI. Along with the reduced lesion size, more preserved neurons were identified in the perilesional area of mice with Ent1 deletion (102 ± 23 in Ent1−/− vs. 73 ± 10 in Ent1+/+). The results of pharmacological inhibition were consistent with the findings of genetic deletion. Moreover, Ent1 inhibition decreased the protein level of complement 3 (an A1 marker), but increased the levels of S100 calcium-binding protein a10 (an A2 marker) and transforming growth factor-β, without changing the levels of inducible nitric oxide synthase (a M1 marker) and arginase 1 (a M2 marker) at the injured site. These findings indicate the important role of Ent1 in the pathogenesis and treatment of SCI.






Similar content being viewed by others
Data Availability
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
References
Brown CM, Mulcahey TA, Filipek NC, Wise PM (2010) Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology 151:4916–4925
David S, Kroner A (2015) Inflammation and secondary damage after spinal cord injury, in Neural Regeneration (So KF and Xu XM eds) pp245–264, Academic Press
Hausmann ON (2003) Post-traumatic inflammation following spinal cord injury. Spinal Cord 41:369–378
Anwar MA, Al Shehabi TS, Eid AH (2016) Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci 10:98
Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle ME, Vernoux N, Tremblay ME et al (2019) Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 10:518
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194
Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834
Rolls A, Shechter R, Schwartz M (2009) The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10:235–241
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967
Diniz LP, Matias IC, Garcia MN, Gomes FC (2014) Astrocytic control of neural circuit formation: highlights on TGF-beta signaling. Neurochem Int 78:18–27
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. N Engl J Med 367:2322–2333
Seydyousefi M, Moghanlou AE, Metz GAS, Gursoy R, Faghfoori MH, Mirghani SJ, Faghfoori Z (2019) Exogenous adenosine facilitates neuroprotection and functional recovery following cerebral ischemia in rats. Brain Res Bull 153:250–256
McAdoo DJ, Robak G, Xu GY, Hughes MG (2000) Adenosine release upon spinal cord injury. Brain Res 854:152–157
Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS (2008) Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28:2033–2042
Irrera N, Arcoraci V, Mannino F, Vermiglio G, Pallio G, Minutoli L, Bagnato G, Anastasi GP et al (2018) Activation of A2A receptor by PDRN reduces neuronal damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal cord injury. Front Pharmacol 9:506
Xu S, Wang J, Zhong J, Shao M, Jiang J, Song J, Zhu W, Zhang F et al (2021) CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin Transl Med 11:e269
Ciruela F, Ferré S, Casadó V, Cortés A, Cunha RA, Lluis C, Franco R (2006) Heterodimeric adenosine receptors: a device to regulate neurotransmitter release. Cell Mol Life Sci 63:2427–2431
Pastor-Anglada M, Pérez-Torras S (2018) Emerging roles of nucleoside transporters. Front Pharmacol 9:606
Ackley MA, Governo RJ, Cass CE, Young JD, Baldwin SA, King AE (2003) Control of glutamatergic neurotransmission in the rat spinal dorsal horn by the nucleoside transporter ENT1. J Physiol 548:507–517
Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, Hawrylycz M, Dang C (2013) Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res 41:D996–D1008
Kao YH, Lin MS, Chen CM, Wu YR, Chen HM, Lai HL, Chern Y, Lin CJ (2017) Targeting ENT1 and adenosine tone for the treatment of Huntington’s disease. Hum Mol Genet 26:467–478
Chang CP, Chang YG, Chuang PY, Nguyen TNA, Wu KC, Chou FY, Cheng SJ, Chen HM et al (2021) Equilibrative nucleoside transporter 1 inhibition rescues energy dysfunction and pathology in a model of tauopathy. Acta Neuropathol Commun 9:112
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635–659
Lai PH, Wang TH, Zhang NY, Wu KC, Yao CJ, Lin CJ (2021) Changes of blood-brain-barrier function and transfer of amyloid beta in rats with collagen-induced arthritis. J Neuroinflammation 18:35
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Boghdadi AG, Teo L, Bourne JA (2020) The neuroprotective role of reactive astrocytes after central nervous system injury. J Neurotrauma 37:681–691
Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD (2010) TGF-beta signaling specifies axons during brain development. Cell 142:144–157
Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K (2000) Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res 85:114–122
Orr MB, Gensel JC (2018) Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15:541–553
Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Ryken TC et al (2013) Pharmacological therapy for acute spinal cord injury. Neurosurgery 72(Suppl 2):93–105
Farr SA, Cuzzocrea S, Esposite E, Campolo M, Niehoff M, Doyle TM, Salvemini D (2020) Adenosine A3 receptor as a novel therapeutic target to reduce secondary events and improve neurocognitive functions following traumatic brain injury. J Neuroinflammation 17:339
Little JW, Ford A, Symons-Liguori AM, Chen Z, Janes K, Doyle T, Xie J, Luongo L et al (2015) Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain 138:28–35
Guitart X, Bonaventura J, Rea W, Orrú M, Cellai L, Dettori I, Pedata F, Brugarolas M et al (2016) Equilibrative nucleoside transporter ENT1 as a biomarker of Huntington disease. Neurobiol Dis 96:47–53
Sari Y, Sreemantula SN, Lee MR, Choi DS (2013) Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci 51:779–787
Lee CC, Chang CP, Lin CJ, Lai HL, Kao YH, Cheng SJ, Chen HM, Liao YP et al (2018) Adenosine augmentation evoked by an ENT1 inhibitor improves memory impairment and neuronal plasticity in the APP/PS1 mouse model of Alzheimer’s disease. Mol Neurobiol 55:8936–8952
Keil GJ, DeLander GE (1995) Time-dependent antinociceptive interactions between opioids and nucleoside transport inhibitors. J Pharmacol Exp Ther 274:1387–1392
Han D, Yu Z, Liu W, Yin D, Pu Y, Feng J, Yuan Y, Huang A et al (2018) Plasma Hemopexin ameliorates murine spinal cord injury by switching microglia from the M1 state to the M2 state. Cell Death Dis 9:1–16
Kwak EK, Kim JW, Kang KS, Lee YH, Hua QH, Park TI, Park JY, Sohn YK (2005) The role of inducible nitric oxide synthase following spinal cord injury in rat. J Korean Med Sci 20:663–669
Hinton DJ, Lee MR, Jang JS, Choi DS (2014) Type 1 equilibrative nucleoside transporter regulates astrocyte-specific glial fibrillary acidic protein expression in the striatum. Brain Behav 4:903–914
Vismara I, Papa S, Veneruso V, Mauri E, Mariani A, De Paola M, Affatato R, Rossetti A et al (2020) Selective modulation of A1 astrocytes by drug-loaded nano-structured gel in spinal cord injury. ACS Nano 14:360–371
Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B et al (2018) Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell Physiol Biochem 50:1535–1559
Neal M, Luo J, Harischandra DS, Gordon R, Sarkar S, Jin H, Anantharam V, Désaubry L et al (2018) Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia 66:137–2157
Abdipranoto-Cowley A, Park JS, Croucher D, Daniel J, Henshall S, Galbraith S, Mervin K, Vissel B (2009) Activin A is essential for neurogenesis following neurodegeneration. Stem Cells 27:1330–1346
Funding
This work was supported in part by Grant no. NTPC110-002 from the New Taipei City Hospital of Taiwan and by Grant no. AS-KPQ-111-KNT from the Academia Sinica.
Author information
Authors and Affiliations
Contributions
K-Y C contributed to the conception and design and funding acquisition; C-S L contributed to the conception and design, acquisition and analysis of data, and manuscript preparation; C-Y P contributed to the conception and design and advised; C-J H, K-C W, H-W Y, and H–L L contributed to the acquisition and analysis of data; Y C contributed to the conception and design and advised; C-J L contributed to the conception and design, advised, manuscript preparation, and funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All the animal experiments were approved by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine (IACUC number: 20200073) and followed the 3Rs principle.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, KY., Lu, CS., Pang, CY. et al. Equilibrative Nucleoside Transporter 1 is a Target to Modulate Neuroinflammation and Improve Functional Recovery in Mice with Spinal Cord Injury. Mol Neurobiol 60, 369–381 (2023). https://doi.org/10.1007/s12035-022-03080-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03080-2