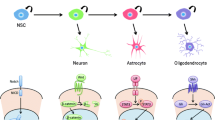
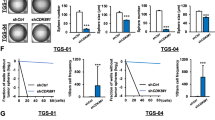
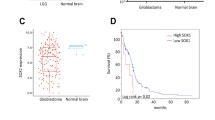
Abstract
Glioma stem cells (GSCs) are thought to drive growth and therapy resistance in glioblastoma (GBM) by “hijacking” at least a subset of signaling pathways active in normal neural stem cells (NSCs). Though the origins of GSCs still remain elusive, uncovering the mechanisms of self-renewing division and cell differentiation in normal NSCs has shed light on their dysfunction in GSCs. However, the distinction between self-renewing division pathways utilized by NSC and GSC becomes critical when considering options for therapeutically targeting signaling pathways that are specifically active or altered in GSCs. It is well-established that cyclin-dependent kinases (CDKs) regulate the cell cycle, yet more recent studies have shown that CDKs also play important roles in the regulation of neuronal survival, metabolism, differentiation, and self-renewal. The intimate relationship between cell cycle regulation and the cellular programs that determine self-renewing division versus cell differentiation is only beginning to be understood, yet seems to suggest potential differential vulnerabilities in GSCs. In this timely review, we focus on the role of CDKs in regulating the self-renewal properties of normal NSCs and GSCs, highlighting novel opportunities to therapeutically target self-renewing signaling pathways specifically in GBM.


Similar content being viewed by others
Data Availability
Not applicable.
References
Flemming W (1965) Contributions to the knowledge of the cell and its vital processes. J Cell Biol 25(1):3–69
Bischoff M, Parfitt DE, Zernicka-Goetz M (2008) Formation of the embryonic-abembryonic axis of the mouse blastocyst: relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Development 135(5):953–962. https://doi.org/10.1242/dev.014316
Piotrowska-Nitsche K, Zernicka-Goetz M (2005) Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev 122(4):487–500. https://doi.org/10.1016/j.mod.2004.11.014
Tabansky I, Lenarcic A, Draft RW, Loulier K, Keskin DB, Rosains J, Rivera-Feliciano J, Lichtman JW et al (2013) Developmental bias in cleavage-stage mouse blastomeres. Curr Biol 23(1):21–31. https://doi.org/10.1016/j.cub.2012.10.054
Burton A, Muller J, Tu S, Padilla-Longoria P, Guccione E, Torres-Padilla ME (2013) Single-cell profiling of epigenetic modifiers identifies PRDM14 as an inducer of cell fate in the mammalian embryo. Cell Rep 5(3):687–701. https://doi.org/10.1016/j.celrep.2013.09.044
Plachta N, Bollenbach T, Pease S, Fraser SE, Pantazis P (2011) Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat Cell Biol 13(2):117–123. https://doi.org/10.1038/ncb2154
Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M (2007) Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445(7124):214–218. https://doi.org/10.1038/nature05458
Conklin EG (1905) Mosaic development in ascidian eggs. J Exp Zool 2(2):145–223. https://doi.org/10.1002/jez.1400020202
Keller GM (1995) In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 7(6):862–869. https://doi.org/10.1016/0955-0674(95)80071-9
Surani MA, Hayashi K, Hajkova P (2007) Genetic and epigenetic regulators of pluripotency. Cell 128(4):747–762. https://doi.org/10.1016/j.cell.2007.02.010
Dhara SK, Stice SL (2008) Neural differentiation of human embryonic stem cells. J Cell Biochem 105(3):633–640. https://doi.org/10.1002/jcb.21891
Mukherjee S, Kong J, Brat DJ (2015) Cancer stem cell division: when the rules of asymmetry are broken. Stem Cells Dev 24(4):405–416. https://doi.org/10.1089/scd.2014.0442
Lewis KM, Petritsch C (2013) Asymmetric cell division: implications for glioma development and treatment. Transl Neurosci 4(4):484–503. https://doi.org/10.2478/s13380-013-0148-8
Lim S, Kaldis P (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Dev 140(15):3079–3093. https://doi.org/10.1242/dev.091744
He S, Nakada D, Morrison SJ (2009) Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 25:377–406. https://doi.org/10.1146/annurev.cellbio.042308.113248
Silva-Vargas V, Delgado AC, Doetsch F (2018) Symmetric Stem Cell Division at the Heart of Adult Neurogenesis. Neuron 98(2):246–248. https://doi.org/10.1016/j.neuron.2018.04.005
Beach D, Durkacz B, Nurse P (1982) Functionally homologous cell cycle control genes in budding and fission yeast. Nature 300(5894):706–709. https://doi.org/10.1038/300706a0
Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T (1983) Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33(2):389–396. https://doi.org/10.1016/0092-8674(83)90420-8
Nurse P, Thuriaux P (1980) Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics 96(3):627–637
Nurse P, Thuriaux P, Nasmyth K (1976) Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 146(2):167–178. https://doi.org/10.1007/BF00268085
Reed SI, Ferguson J, Groppe JC (1982) Preliminary characterization of the transcriptional and translational products of the Saccharomyces cerevisiae cell division cycle gene CDC28. Mol Cell Biol 2(4):412–425. https://doi.org/10.1128/mcb.2.4.412
Sherr CJ (1994) G1 phase progression: cycling on cue. Cell 79(4):551–555. https://doi.org/10.1016/0092-8674(94)90540-1
Sherr CJ (1996) Cancer cell cycles. Sci 274(5293):1672–1677. https://doi.org/10.1126/science.274.5293.1672
Pavletich NP (1999) Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 287(5):821–828. https://doi.org/10.1006/jmbi.1999.2640
Shah K, Lahiri DK (2014) Cdk5 activity in the brain - multiple paths of regulation. J Cell Sci 127(Pt 11):2391–2400. https://doi.org/10.1242/jcs.147553
Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ (2008) Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A 105(47):18567–18571. https://doi.org/10.1073/pnas.0810137105
Shupp A, Casimiro MC, Pestell RG (2017) Biological functions of CDK5 and potential CDK5 targeted clinical treatments. Oncotarget 8(10):17373–17382. https://doi.org/10.18632/oncotarget.14538
Lew J, Beaudette K, Litwin CM, Wang JH (1992) Purification and characterization of a novel proline-directed protein kinase from bovine brain. J Biol Chem 267(19):13383–13390. https://doi.org/10.1016/S0021-9258(18)42222-3
Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461(7266):947–955. https://doi.org/10.1038/nature08435
Costa MR, Wen G, Lepier A, Schroeder T, Gotz M (2008) Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Dev 135(1):11–22. https://doi.org/10.1242/dev.009951
Morales AV, Mira H (2019) Adult Neural Stem Cells: Born to Last. Front Cell Dev Biol 7:96. https://doi.org/10.3389/fcell.2019.00096
Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Hanecker P, Ayers-Ringler J et al (2011) Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell 20(3):328–340. https://doi.org/10.1016/j.ccr.2011.08.011
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Beck B, Blanpain C (2013) Unravelling cancer stem cell potential. Nat Rev Cancer 13(10):727–738. https://doi.org/10.1038/nrc3597
Hitomi M, Chumakova AP, Silver DJ, Knudsen AM, Pontius WD, Murphy S, Anand N, Kristensen BW et al (2021) Asymmetric cell division promotes therapeutic resistance in glioblastoma stem cells. JCI Insight 6 (3). https://doi.org/10.1172/jci.insight.130510
Lathia JD, Hitomi M, Gallagher J, Gadani SP, Adkins J, Vasanji A, Liu L, Eyler CE et al (2011) Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis 2:e200. https://doi.org/10.1038/cddis.2011.80
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414(6859):105–111. https://doi.org/10.1038/35102167
Xiang H, Yuan L, Gao X, Alexander PB, Lopez O, Lau C, Ding Y, Chong M et al (2017) UHRF1 is required for basal stem cell proliferation in response to airway injury. Cell Discov 3:17019. https://doi.org/10.1038/celldisc.2017.19
Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, Um JY, Kim WK et al (2018) Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 560(7717):243–247. https://doi.org/10.1038/s41586-018-0389-3
Yamaki T, Shibahra I, Matsuda KI, Kanemura Y, Konta T, Kanamori M, Yamakawa M, Tominaga T et al (2020) Relationships between recurrence patterns and subventricular zone involvement or CD133 expression in glioblastoma. J Neurooncol 146(3):489–499. https://doi.org/10.1007/s11060-019-03381-y
Kwan K, Schneider JR, Patel NV, Boockvar JA (2019) Tracing the Origin of Glioblastoma: Subventricular Zone Neural Stem Cells. Neurosurg 84(1):E15-e16. https://doi.org/10.1093/neuros/nyy512
Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y (2009) Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell 15(6):514–526. https://doi.org/10.1016/j.ccr.2009.04.001
Daniel PM, Filiz G, Brown DV, Christie M, Waring PM, Zhang Y, Haynes JM, Pouton C et al (2018) PI3K activation in neural stem cells drives tumorigenesis which can be ameliorated by targeting the cAMP response element binding protein. Neuro Oncol 20(10):1344–1355. https://doi.org/10.1093/neuonc/noy068
Abel TW, Clark C, Bierie B, Chytil A, Aakre M, Gorska A, Moses HL (2009) GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol Cancer Res 7(5):645–653. https://doi.org/10.1158/1541-7786.Mcr-08-0477
Duan S, Yuan G, Liu X, Ren R, Li J, Zhang W, Wu J, Xu X et al (2015) PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype. Nat Commun 6(1):10068. https://doi.org/10.1038/ncomms10068
Zhang G-L, Wang C-F, Qian C, Ji Y-X, Wang Y-Z (2021) Role and mechanism of neural stem cells of the subventricular zone in glioblastoma. World J Stem Cells 13(7):877–893. https://doi.org/10.4252/wjsc.v13.i7.877
Gimple RC, Bhargava S, Dixit D, Rich JN (2019) Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev 33(11–12):591–609. https://doi.org/10.1101/gad.324301.119
Piper K, DePledge L, Karsy M, Cobbs C (2021) Glioma Stem Cells as Immunotherapeutic Targets: Advancements and Challenges. Frontiers Oncol 11. https://doi.org/10.3389/fonc.2021.615704
Calegari F, Haubensak W, Haffner C, Huttner WB (2005) Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci 25(28):6533–6538. https://doi.org/10.1523/JNEUROSCI.0778-05.2005
Lange C, Huttner WB, Calegari F (2009) Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5(3):320–331. https://doi.org/10.1016/j.stem.2009.05.026
Chirivella L, Kirstein M, Ferron SR, Domingo-Muelas A, Durupt FC, Acosta-Umanzor C, Cano-Jaimez M, Perez-Sanchez F et al (2017) Cyclin-dependent kinase 4 regulates adult neural stem cell proliferation and differentiation in response to insulin. Stem Cells 35(12):2403–2416. https://doi.org/10.1002/stem.2694
Calegari F, Huttner WB (2003) An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci 116(Pt 24):4947–4955. https://doi.org/10.1242/jcs.00825
Pevny LH, Nicolis SK (2010) Sox2 roles in neural stem cells. Int J Biochem Cell Biol 42(3):421–424. https://doi.org/10.1016/j.biocel.2009.08.018
Lim S, Bhinge A, Bragado Alonso S, Aksoy I, Aprea J, Cheok CF, Calegari F, Stanton LW, Kaldis P (2017) Cyclin-dependent kinase-dependent phosphorylation of Sox2 at serine 39 regulates neurogenesis. Mol Cell Biol 37 (16). https://doi.org/10.1128/MCB.00201-17
Ali F, Hindley C, McDowell G, Deibler R, Jones A, Kirschner M, Guillemot F, Philpott A (2011) Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis. Dev 138(19):4267–4277. https://doi.org/10.1242/dev.067900
Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M et al (2016) Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Life 5:e13374. https://doi.org/10.7554/eLife.13374
Wang J, Huang Y, Cai J, Ke Q, Xiao J, Huang W, Li H, Qiu Y et al (2018) A nestin-cyclin-dependent kinase 5-dynamin-related protein 1 axis regulates neural stem/progenitor cell stemness via a metabolic shift. Stem Cells 36(4):589–601. https://doi.org/10.1002/stem.2769
Zheng YL, Li BS, Rudrabhatla P, Shukla V, Amin ND, Maric D, Kesavapany S, Kanungo J et al (2010) Phosphorylation of p27Kip1 at Thr187 by cyclin-dependent kinase 5 modulates neural stem cell differentiation. Mol Biol Cell 21(20):3601–3614. https://doi.org/10.1091/mbc.E10-01-0054
Altea-Manzano P, Cuadros AM, Broadfield LA, Fendt SM (2020) Nutrient metabolism and cancer in the in vivo context: a metabolic game of give and take. EMBO Rep 21(10):e50635. https://doi.org/10.15252/embr.202050635
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y (2021) Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 6(1):263. https://doi.org/10.1038/s41392-021-00658-5
Dundar B, Markwell SM, Sharma NV, Olson CL, Mukherjee S, Brat DJ (2020) Methods for in vitro modeling of glioma invasion: choosing tools to meet the need. Glia 68(11):2173–2191. https://doi.org/10.1002/glia.23813
Kreso A, Dick JE (2014) Evolution of the cancer stem cell model. Cell Stem Cell 14(3):275–291. https://doi.org/10.1016/j.stem.2014.02.006
Brooks MD, Burness ML, Wicha MS (2015) Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell 17(3):260–271. https://doi.org/10.1016/j.stem.2015.08.014
Hambardzumyan D, Bergers G (2015) Glioblastoma: defining tumor niches. Trends Cancer 1(4):252–265. https://doi.org/10.1016/j.trecan.2015.10.009
Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S et al (2017) Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32(1):42-56.e46. https://doi.org/10.1016/j.ccell.2017.06.003
Yang X, Xiao Z, Du X, Huang L, Du G (2017) Silencing of the long non-coding RNA NEAT1 suppresses glioma stem-like properties through modulation of the miR-107/CDK6 pathway. Oncol Rep 37(1):555–562. https://doi.org/10.3892/or.2016.5266
Jung J, Gilbert MR, Park DM (2016) Isolation and propagation of glioma stem cells from acutely resected tumors. Methods Mol Biol 1516:361–369. https://doi.org/10.1007/7651_2016_342
Codrici E, Enciu AM, Popescu ID, Mihai S, Tanase C (2016) Glioma stem cells and their microenvironments: providers of challenging therapeutic targets. Stem Cells Int 2016:5728438. https://doi.org/10.1155/2016/5728438
Liebelt BD, Shingu T, Zhou X, Ren J, Shin SA, Hu J (2016) Glioma stem cells: signaling, microenvironment, and therapy. Stem Cells Int 2016:7849890. https://doi.org/10.1155/2016/7849890
Kim SS, Pirollo KF, Chang EH (2015) Isolation and culturing of glioma cancer stem cells. Curr Protoc Cell Biol 67(23):10 21-23 10 10. https://doi.org/10.1002/0471143030.cb2310s67
Beier D, Schriefer B, Brawanski K, Hau P, Weis J, Schulz JB, Beier CP (2012) Efficacy of clinically relevant temozolomide dosing schemes in glioblastoma cancer stem cell lines. J Neurooncol 109(1):45–52. https://doi.org/10.1007/s11060-012-0878-4
Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488(7412):522–526. https://doi.org/10.1038/nature11287
Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE, Hjelmeland AB, Huang AY et al (2011) Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One 6(9):e24807. https://doi.org/10.1371/journal.pone.0024807
Li X, Martinez-Ledesma E, Zhang C, Gao F, Zheng S, Ding J, Wu S, Nguyen N et al (2019) Tie2-FGFR1 interaction induces adaptive PI3K inhibitor resistance by upregulating Aurora A/PLK1/CDK1 signaling in glioblastoma. Cancer Res 79(19):5088–5101. https://doi.org/10.1158/0008-5472.CAN-19-0325
Li M, Xiao A, Floyd D, Olmez I, Lee J, Godlewski J, Bronisz A, Bhat KPL et al (2017) CDK4/6 inhibition is more active against the glioblastoma proneural subtype. Oncotarget 8(33):55319–55331. https://doi.org/10.18632/oncotarget.19429
Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z et al (2015) Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci 18(4):501–510. https://doi.org/10.1038/nn.3960
Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS et al (2014) Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 157(3):580–594. https://doi.org/10.1016/j.cell.2014.02.030
Mukherjee S, Tucker-Burden C, Kaissi E, Newsam A, Duggireddy H, Chau M, Zhang C, Diwedi B et al (2018) CDK5 inhibition resolves PKA/cAMP-independent activation of CREB1 signaling in glioma stem cells. Cell Rep 23(6):1651–1664. https://doi.org/10.1016/j.celrep.2018.04.016
Ning JF, Stanciu M, Humphrey MR, Gorham J, Wakimoto H, Nishihara R, Lees J, Zou L et al (2019) Myc targeted CDK18 promotes ATR and homologous recombination to mediate PARP inhibitor resistance in glioblastoma. Nat Commun 10(1):2910. https://doi.org/10.1038/s41467-019-10993-5
Xie Q, Wu Q, Kim L, Miller TE, Liau BB, Mack SC, Yang K, Factor DC et al (2016) RBPJ maintains brain tumor-initiating cells through CDK9-mediated transcriptional elongation. J Clin Invest 126(7):2757–2772. https://doi.org/10.1172/JCI86114
Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN (2008) c-Myc is required for maintenance of glioma cancer stem cells. PLoS One 3(11):e3769. https://doi.org/10.1371/journal.pone.0003769
Fukasawa K, Kadota T, Horie T, Tokumura K, Terada R, Kitaguchi Y, Park G, Ochiai S et al (2021) CDK8 maintains stemness and tumorigenicity of glioma stem cells by regulating the c-MYC pathway. Oncogene 40(15):2803–2815. https://doi.org/10.1038/s41388-021-01745-1
Dews M, Fox JL, Hultine S, Sundaram P, Wang W, Liu YY, Furth E, Enders GH et al (2010) The myc-miR-17~92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors. Can Res 70(20):8233–8246. https://doi.org/10.1158/0008-5472.CAN-10-2412
Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, Luthra S, Chandran UR et al (2013) Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A 110(21):8644–8649. https://doi.org/10.1073/pnas.1221478110
Juric V, Murphy B (2020) Cyclin-dependent kinase inhibitors in brain cancer: current state and future directions. Cancer Drug Resist 3(1):48–62. https://doi.org/10.20517/cdr.2019.105
Lubanska D, Porter L (2017) Revisiting CDK inhibitors for treatment of glioblastoma multiforme. Drugs R D 17(2):255–263. https://doi.org/10.1007/s40268-017-0180-1
Le Rhun E, von Achenbach C, Lohmann B, Silginer M, Schneider H, Meetze K, Szabo E, Weller M (2019) Profound, durable and MGMT-independent sensitivity of glioblastoma cells to cyclin-dependent kinase inhibition. Int J Cancer 145(1):242–253. https://doi.org/10.1002/ijc.32069
Su Y-T, Chen R, Wang H, Song H, Zhang Q, Chen L-Y, Lappin H, Vasconcelos G et al (2018) Novel targeting of transcription and metabolism in glioblastoma. Clin Cancer Res 24(5):1124–1137. https://doi.org/10.1158/1078-0432.CCR-17-2032
Wu J, Yuan Y, Long Priel DA, Fink D, Peer CJ, Sissung TM, Su Y-T, Pang Y et al (2021) Phase I study of zotiraciclib in combination with temozolomide for patients with recurrent high-grade astrocytomas. Clin Cancer Res 27(12):3298–3306. https://doi.org/10.1158/1078-0432.CCR-20-4730
Lee's Pharmaceutical L (2020) Phase I clinical study of oral TG02 capsule in the treatment of recurrent progressive high-grade glioma patients. https://ClinicalTrials.gov/show/NCT03904628.
National Cancer I, National Institutes of Health Clinical C (2020) Zotiraciclib (TG02) Plus dose-dense or metronomic temozolomide followed by randomized phase II trial of zotiraciclib (TG02) plus temozolomide versus temozolomide alone in adults with recurrent anaplastic astrocytoma and glioblastoma. https://ClinicalTrials.gov/show/NCT02942264.
European Organisation for R, Treatment of Cancer E, Tragara Pharmaceuticals I (2022) Study of TG02 in elderly newly diagnosed or adult relapsed patients with anaplastic astrocytoma or glioblastoma. https://ClinicalTrials.gov/show/NCT03224104.
Ranjan A, Pang Y, Butler M, Merchant M, Kim O, Yu G, Su Y-T, Gilbert MR et al (2021) Targeting CDK9 for the treatment of glioblastoma. Cancers (Basel) 13(12):3039. https://doi.org/10.3390/cancers13123039
Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M et al (2016) Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 6(7):740–753. https://doi.org/10.1158/2159-8290.Cd-16-0095
Noonan JJ, Jarzabek M, Lincoln FA, Cavanagh BL, Pariag AR, Juric V, Young LS, Ligon KL et al (2019) Implementing patient-derived xenografts to assess the effectiveness of cyclin-dependent kinase inhibitors in glioblastoma. Cancers (Basel) 11 (12). https://doi.org/10.3390/cancers11122005
Juric V, Düssmann H, Lamfers MLM, Prehn JHM, Rehm M, Murphy BM (2021) Transcriptional CDK Inhibitors CYC065 and THZ1 induce apoptosis in glioma stem cells derived from recurrent GBM. Cells 10 (5). https://doi.org/10.3390/cells10051182
Poratti M, Marzaro G (2019) Third-generation CDK inhibitors: a review on the synthesis and binding modes of Palbociclib, Ribociclib and Abemaciclib. Eur J Med Chem 172:143–153. https://doi.org/10.1016/j.ejmech.2019.03.064
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, Yu M, Lin J et al (2020) The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci 21 (6). https://doi.org/10.3390/ijms21061960
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15(6):122. https://doi.org/10.1186/gb4184
Lai L, Shin GY, Qiu H (2020) The role of cell cycle regulators in cell survival-dual functions of cyclin-dependent kinase 20 and p21(Cip1/Waf1). Int J Mol Sci 21 (22). https://doi.org/10.3390/ijms21228504
Funding
This work is supported by NIH grant R01CA214928.
Author information
Authors and Affiliations
Contributions
LKS and SM are equal contributors.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Research Involving Human Participants and/or Animals
Not applicable.
Other
All figures and tables were created with BioRender.com.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shih, LK., Mukherjee, S. & Brat, D.J. Off the Clock: the Non-canonical Roles of Cyclin-Dependent Kinases in Neural and Glioma Stem Cell Self-Renewal. Mol Neurobiol 59, 6805–6816 (2022). https://doi.org/10.1007/s12035-022-03009-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03009-9