Abstract
The unique contribution of the serotonin transporter-linked polymorphic region (5-HTTLPR), intronic region 2 (STin2), and monoamine oxidase A (MAO-A) genes to individual differences in personality traits has been widely explored, and research has shown that certain forms of these polymorphisms relate to impulsivity and impulsivity-related disorders. Humans showing these traits are also described as having an asymmetrical prefrontal cortical activity when compared to others. In this explorative study, we examine the relationship between serotonergic neurotransmission polymorphisms, cortical activity features (prefrontal alpha asymmetry, individual alpha peak frequency [iAPF]), emotion-related and non-emotion-related impulsivity in humans. 5-HTTLPR, MAO-A, and STin2 polymorphisms were assessed in blood taken from 91 participants with high emotion-related impulsivity levels. Sixty-seven participants completed resting electroencephalography and a more comprehensive impulsivity index. In univariate analyses, iAPF correlated with both forms of emotion-related impulsivity. In multiple linear regression models, 5-HTTLPR polymorphism (model 1, adj. R2 = 15.2%) and iAPF were significant interacting predictors of emotion-related impulsivity, explaining a large share of the results’ variance (model 2, adj. R2 = 21.2%). Carriers of the low transcriptional activity 5-HTTPLR and MAO-A phenotypes obtained higher emotion-related impulsivity scores than others did. No significant results were detected for non-emotion-related impulsivity or for a form of emotion-related impulsivity involving cognitive/motivational reactivity to emotion. Our findings support an endophenotypic approach to impulsivity, showing that tri-allelic 5-HTTLPR polymorphism, iAPF, and their interaction are relevant predictors of one form of emotion-related impulsivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impulsivity is a multidimensional personality construct associated with a wide range of psychological disorders (e.g., major depressive disorder, bipolar disorder, attention-deficit hyperactivity disorder, suicide) [1,2,3,4,5]. While older impulsivity definitions tended to focus on problems with planning, deliberation, and attention [6, 7], newer research reports the importance of impulsivity in response to states of high positive or negative emotions [2, 8, 9]. Emotion-related impulsivity has been defined as the reflexive tendency to act impulsively during periods of heightened emotion [9]. A large body of work shows that emotion-related impulsivity is more robustly tied to psychopathologies, aggression, and suicide than are other forms of impulsivity [10, 11].
Serotonergic neurotransmission modulates mood and emotion [12, 13], consequently affecting a wide spectrum of impulsivity-related traits. Two key regulators of serotonergic signaling are the serotonin transporter (5-HTT) and the monoamine oxidase A (MAO-A), which respectively remove serotonin from the synaptic cleft and catabolize monoamines with a strong affinity for serotonin and catecholamines [13,14,15]. The 5-HTT protein is encoded by the gene SLC6A4, whose transcriptional activity is modulated by several variations, including two repetitive sequences called the serotonin transporter-linked polymorphic region (5-HTTLPR) and the serotonin transporter intronic region 2 (STin2) variable number tandem repeat (VNTR) [16]. Likewise, the MAO-A gene has a VNTR polymorphism modulating its transcription. Transcriptional activity levels in each of these monoamine genes result in different expression rates of their respective mRNA and subsequent proteins and can therefore be classified in phenotypic categories (Fig. 1) [14, 15, 17, 18]. These phenotypic distinctions are also related to some psychological traits, especially impulsivity [14, 15].
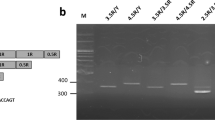
Allelic variations for serotonin transporter linked polymorphic region (5-HTTLPR), serotonin transporter intronic region 2 (STin2), and monoamine oxidase A (MAO-A) leading to different transcriptional activity phenotypes. Only the most frequent alleles are illustrated in A. Chrom., chromosome; 5-HTT, serotonin transporter; 5-HT, serotonin; rep., repeats; VNTR, variable number tandem repeat. This figure has been created using BioRender.com. B Phenotype categorization used in this study based on genes’ transcriptional activity levels and their occurrence frequency (in percentage) in this analysis. MAO-A being located on chromosome X, male participants comport only one allele of the gene. All results were concordant with participants’ gender. The phenotype categorization was realized using the following references: [14, 15, 17, 18]. S, short; L, long; a, adenine; g, guanine
More specifically, these polymorphisms have been associated with impulsivity and impulsivity-related disorders. For example, impulsivity [9], neuroticism [14], anxiety [19], and emotional instability [14] are more frequent in people having the low 5-HTTLPR transcriptional activity (5-HTTLPRLow) phenotype. The shortest repetitive sequences of STin2 are associated with elevated cognitive impulsivity [20], anxiety scores [21], major depressive disorders [22], early-onset bipolar disorder [23], and suicide attempts [24]. Finally, MAO-A phenotypes are linked to antisocial behaviors [15], emotional instability [25], impulsivity [26], bipolar disorders [27], and violent aggression [28].
Attempts to understand the neurogenetic basis of impulsivity have been limited by the relative absence of attention to the multidimensional nature of impulsivity. One previous study suggested that emotion-related impulsivity, but not non-emotion-related impulsivity, was tied to 5-HTTLPR [9]. Therefore, the aim of this explorative study was to evaluate serotonergic genetic markers while differentiating between emotion-related and non-emotion-related impulsivity.
Beyond these genetic markers, we consider other stable genetically modulated variables to provide a more comprehensive understanding of the determinants of impulsivity. Cortical activity, including lateralization of frontal oscillations in the alpha frequency band, is a partially genetically modulated parameter sometimes found to be associated with genetic polymorphisms such as the 5-HTTLPRLow phenotype [29]. For more than 50 years, research has demonstrated that frontocortical regions are asymmetrically related to motivational and emotional variables, such as approach and avoidance tendencies: heightened relative activity in the left frontal cortex is related to approach motivation, whereas heightened relative activity in the right frontal cortex is related to avoidance motivation [30,31,32,33]. Impulsivity (in particular, its urgency and positive urgency dimensions) can be depicted as the inability to inhibit approach urges [34, 35]. Drawing on these findings, multiple investigators have shown that greater left prefrontal activity during rest (i.e., a right-sided predominance of alpha power as alpha oscillations are inversely related to cortical activity [36]) is associated with impulsivity [37,38,39].
There is reason to believe that emotion-related impulsivity may be more closely tied to lateralization indices than non-emotion-related impulsivity is. High emotional instability has frequently been associated with lateralization of the frontal alpha activity [29, 37,38,39,40]. Regions of the left prefrontal cortex are believed to play an important role in inhibiting the amygdala [41, 42], and their activation leads to downregulation of the amygdala when participants are asked to downregulate negative affect [43, 44]. Accordingly, we hypothesize that persons exhibiting highly impulsive responses to emotion may show stronger right alpha prefrontal activity than others.
Other cortical activity characteristics, such as individual alpha peak frequency (iAPF), are highly heritable, appear to be under substantial genetic control [45,46,47], and show high stability over test–retest intervals in healthy and clinical conditions [48, 49]. Given this, iAPF is considered to be a valuable marker for understanding psychological traits [50]. Furthermore, seminal research has already shown that alpha power and alpha peak frequency were higher in highly impulsive individuals when compared to low impulsive individuals [51]. Compared to the alpha power, the iAPF has more robust heritability estimates and better test–retest reliability [48, 52].
Thus, in the current study, we used an endophenotypic approach, combining genetic and cortical features to better understand the mechanisms underlying emotion-related impulsivity. Serotonergic neurotransmission polymorphisms (i.e., 5-HTTLPR, STin2, and MAO-A), prefrontal alpha asymmetry, and iAPF were assessed in emotionally impulsive humans. We hypothesized that the low transcriptional activity phenotypes in serotonergic neurotransmission polymorphisms and high right prefrontal alpha asymmetry would relate to higher emotion-related impulsivity. According to seminal research, the iAPF should be higher in highly impulsive individuals when compared to others.
Material and Methods
All samples and data used in this explorative study were taken at the baseline testing of an intervention study called NoSTRESS. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was registered on the German Clinical Trial registration website (DRKS00016589) and approved by the university review board before data collection.
Participants
Participants were recruited through advertising on the university website and online social networks (i.e., Facebook, Twitter, and LinkedIn) based on high scores on a measure of emotion-related impulsivity. As presented in Fig. 2, 91 participants (one participant later excluded because of insufficient DNA quality) were included in genotyping step (mean age = 29.9 ± 7.7). Sixty-seven participants attended a second appointment where the resting electroencephalogram (EEG) and fuller impulsivity measurements were gathered (mean age = 30.3 ± 7.6). Details are presented in Fig. 2.
Experimental Design
Participants completed consent, a self-report measure of emotion-related impulsivity (Feelings Trigger Action) [9], and questions on inclusion/exclusion criteria via the Qualtrics platform. The 35% of participants with the highest emotion-related impulsivity levels were invited to the laboratory to perform a quasi-ramp cardiopulmonary exercise testing (CPET; not relevant here but available in [53]) and blood testing between 7 and 11 a.m. and in a fasted state (8–16 h).
The exclusion and inclusion criteria were double-checked at the lab, and written informed consent was gathered. A short medical check-up was conducted. Blood was drawn via venipuncture at rest. On a separate day within the same week, participants were invited to a second appointment to record resting cortical activity and complete a broader index of impulsivity, the Three-Factor Impulsivity index [9].
Inclusion/Exclusion Criteria
Exclusion criteria were designed for the NoSTRESS study [53] and included current pregnancy, breastfeeding, specific medical conditions or psychological disorders diagnosed by clinicians, use of antidepressant medication, engagement in more than three hours of exercise per week, and fitness level (i.e., VO2peak) at or below fair (age and gender corrected, based on the Standards of the Federal Office for Sport [Bundesamtes für Sport]). Participants had to be native German speakers, between 18 and 50 years old, and among the 35% highest emotion-related impulsivity scores [53] (DRKS00016589).
DNA Isolation
Blood samples were taken in K2 EDTA tubes, and buffy coats were collected after two centrifugation cycles—phosphate-buffered saline (PBS) re-suspension. Freezing medium was added to the samples (1 mL for 300 µL of buffy coat) that were stored at − 150 °C until study completion. On the day of analysis, the buffy coat was brought to room temperature and then centrifuged for 10 min at 5500 rpm. The supernatant was discarded. The pellet was re-suspended with a 200 µL PBS solution. DNA was isolated using Blood DNA Mini Kit (Bio-Budget, Technologies GmbH, Germany). Quantification and quality assessment of the DNA were performed using a single sample spectrophotometer (NanoDrop 1000, peqlab, biotechnologie, GmbH). Samples were stored at − 20 °C until required.
Genotyping
Primers were synthesized by Invitrogen, Thermo Fisher Scientific, Germany (Supplementary Material A) based on former uses [54,55,56]. Participant’s genomic DNA (20 ng) was amplified using the premixed ready-to-use solution GoTaq Colorless MasterMix (Promega, Madison, USA) and equimolar concentrations (50 pmol final concentration) of forward and reverse primers for 5-HTTLPR, STin2, and MAO-A (Invitrogen, Thermo Fisher Scientific, Germany). Complete polymerase chain reaction (PCR) cycling conditions for each gene are described in Supplementary Material A. Annealing temperatures were determined using temperature gradient (63° for MAO-A and STin2, 61° for 5-HTTLPR). To determine the presence of the rs25531 single nucleotide polymorphism within the 5-HTTLPR region, a restriction enzyme digest was performed on the PCR amplicons. Twenty microliters of the product was digested with 1 µL MspI (New England Biolabs, Herts, UK) at 37 °C for 6 h. PCR amplicons were separated by 0.5 × tris–borate-EDTA-buffered 1.5% agarose gel (loading dye without sodium dodecyl sulfate) and visualized with ethidium bromide using a UV trans-illuminator. All samples were duplicated for assay reliability. Interpretation of the PCR products are presented in Supplementary Material A. Phenotype and genotype occurrences are presented, respectively, in Fig. 1B and Supplementary Material A.
Three-Factor Impulsivity Index [9]
The Three-Factor Impulsivity index is a 54-item composite self-report measure of impulsivity. Items are rated from 1 (“I strongly disagree”) to 5 (“I strongly agree”), with higher scores reflecting higher impulsivity levels. The questionnaire covers eight different components of impulsivity shown in oblique factor analyses and confirmatory structural equation modeling to load onto three separate factors [9, 57]: Pervasive Influence of Feelings, (lack of) Follow-Through, and Feelings Trigger Action; alphas = 0.837, 0.897, and 0.857, respectively. The first and the third factors are emotion-related. Factor one, Pervasive Influence of Feelings, reflects unconstrained cognitive and motivational responses to negative emotions and includes items from previously validated scales for negative generalization [58], negative urgency [8], and novel items to capture lethargy in response to sadness and extremely negative thoughts of self and the world in response to emotions. Factor two, (lack of) Follow-Through, includes items from previously validated scales designed to cover distractibility and lack of perseverance [8]. The third factor, Feelings Trigger Action, which was used for screening, includes items from the previously validated scales of Negative Urgency [8], Positive Urgency [59], and items to capture responding reflexively and quickly when experiencing emotions. The German validated version of the questionnaire was used [60]. One attention catch item (“Please select I agree”) was embedded in the online screening questionnaire, and two such items in the full Three-Factor Impulsivity index. All participants answered the three catch items properly, and no data were removed for failing these items. Means and standard deviation were 3.19 ± 0.71 for Pervasive Influence of Feelings (n = 67), 2.80 ± 0.59 for (lack of) Follow-Through (n = 67), 3.11 ± 0.44 for Feelings Trigger Action at T0 (n = 67), and 3.22 ± 0.35 for Feeling Trigger Action at screening (n = 91).
Electroencephalography
Data Acquisition
The EEG was recorded continuously for 5 min while participants sat alone in a relaxed position with eyes closed in a testing room with temperature and humidity maintained at 20.5 ± 0.5 °C and 44 ± 11%, respectively. A BioSemi Active-Two system (BioSemi, Amsterdam, Netherlands) with 64 Ag/AgCl electrodes embedded in an elastic cap and placed according to the international 10–20 system was used. The system records the voltage between each electrode and an active common mode sense (CMS) electrode that forms a feedback loop with a passive drive right leg (DLR) electrode. CMS and DLR were located in parieto-occipital positions. The sampling frequency was set at 2048 Hz, and the electrodes’ offset was kept below 50 µV. ActiView software (BioSemi, Amsterdam, Netherlands) was used to record the data.
Data Analysis
Offline EEG data processing was conducted using Python’s (v3.8.5) MNE package (v0.22.0) [61]. First, power line noise at 50 Hz and its respective harmonics was attenuated by notch filters (overlap-add finite impulse response filtering). Then, the data were re-referenced to the average reference. Bad channels were detected automatically using the noisy channel detection algorithm of pyprep (per deviation; threshold = 5z) [62] and via visual inspection. If bad channels were detected, they were subsequently removed, and data were interpolated using spherical splines as long as three original neighboring signals were available for interpolation. Muscle artifacts were automatically detected and annotated within the continuous raw data using the MNE annotate_muscle_zscore method. Then, the raw data were filtered with a 1-Hz high-pass and 40-Hz low-pass filter (both overlap-add finite impulse response filtering). Power spectra density was computed by Welch’s method using 1-s segments with 50% overlap. Segments containing previously annotated muscle artifacts and a peak-to-peak amplitude exceeding 200 µV in any channel were rejected. On average across participants, 533 ± 47 quality-sufficient epochs were used in the analysis.
Scientific literature provides a spectrum of studies linking impulsivity to different frontal cortex regions, e.g., [31,32,33, 37, 39, 63,64,65]. Thus, we used a comprehensive approach going from the general to the specific. In preliminary analyses, we have first tested the frontal cortex, then the prefrontal cortex, and finally specific pairs of electrodes from the prefrontal region often used in asymmetry literature: Fp2/Fp1, F2/F1, F4/F3, and F8/F7 (details in Supplementary Material A). These multiple tests were adjusted using a family-wise Bonferroni correction (see “Statistics” section). As suggested in previous research [29], the alpha asymmetry was indexed using laterality coefficients (LC) using the following formula: LC = (power right – power left) / (power right + power left) × 100. Values superior to zero indicate higher alpha activity in the right cortex compared to the left one, in other words, a greater left cortical activity. LC has been used for a long time in the field of laterality because it separates the asymmetry variance from the general magnitude variance [64]. This score is perfectly correlated with another metric commonly reported in EEG studies (ln (right) – ln (left)) [66]. Nevertheless, using the LC allows easier comparison between different studies, different frequency bands, and locations [67].
IAPF was estimated from the power spectra densities computed from 1-s non-overlapping segments that were zero-padded to 10 s to have a frequency resolution of 0.1 Hz. The iAPF between 8 and 13 Hz was determined from the mean over 17 posterior electrodes (Pz, P1/2, P3/4, P5/6, P7/8, POz, PO3/4, PO7/8, Oz, and O1/2) [50].
Statistics
Statistical analyses were conducted using R (v1.2.1335) and SPSS (v23). The dataset and the R script are provided in Supplementary Materials B and C. Data were first z-standardized (for dependant variables and continuous predictors) and then winsorized at ± 3z [68]. The distribution of each variable was evaluated using the Shapiro–Wilk test and checked for linearity (via quantile–quantile plots and histograms of standardized residuals), skewness, and kurtosis. Pearson’s bivariate correlations were used to evaluate the relationship of EEG markers with impulsivity levels. As the alpha asymmetry was evaluated using six parameters, a Bonferroni alpha correction was applied family-wise by setting the significance level at p < 0.008. All univariate analyses were controlled for age and gender.
Multiple linear regression models were used to assess how gene phenotypes, individual alpha frequency, and cortical asymmetry LC contributed to impulsivity (continuous scores). The predictors were either categorical (i.e., phenotypes, gender) or continuous (i.e., iAPF, LCs, age) [68]. As the MAO-A gene is located on the X chromosome, and impulsivity can vary with gender, gender was included as a potential predictor. Because impulsivity and iAPF have been shown to change with age, age was included as a predictor [50, 69]. To avoid potential collinearity, we included F4/F3, the most commonly used asymmetry marker, as the only pre-frontal-asymmetry marker. Based on the literature, one can expect interaction between 5-HTTLPR polymorphisms and cortical activity [29]. Thus, these interaction terms were included in the regression models. Missing values were imputed based on the mean when inferior to 10% of the total number of values (F4/F3 LC: 4.5% and iAPF: 8.9%) [70, 71].
All predictors were checked for multicollinearity (variance inflation factor and tolerance values were acceptable at < 2 and > 0.2, respectively), independence (Durbin Watson test was acceptable, results ranged from 1 to 3), and linearity (graphically via quantile–quantile plot, scatterplot, and histogram of studentized residuals) [72]. All assumptions for the statistical analysis were met. As models 1 and 2 both assessed Feeling Trigger Action, a Bonferroni alpha correction was applied, bringing the significance level to p < 0.025. For the other parameters, the significance level was set to p < 0.050.
Results
Univariate Analyses
Impulsivity and Polymorphisms
Average impulsivity scores per gene and transcriptional activity phenotypes are reported in Supplementary Material A. When controlled for age and gender, Feelings Trigger Action levels at screening differed significantly among 5-HTTLPR transcriptional activity phenotypes F(2, 85) = 8.853, p < 0.001, np2 = 0.172 and at T0 F(2, 62) = 3.220, p < 0.050, np2 = 0.094. Post hoc analyses revealed that carriers of the 5-HTTLPRLow phenotype had significantly higher Feelings Trigger Action scores than the moderate (5HTTLPRModerate) and high transcriptional activities (5-HTTLPRHigh) phenotype carriers. Moreover, Feelings Trigger Action scores were significantly higher for the low MAO-A VNTR transcriptional activity (MAO-ALow) phenotype carriers compared to the high ones (MAO-AHigh), F(1, 63) = 4.927, p < 0.050, np2 = 0.073 at T0, but were only a trend at screening, F(1, 86) = 3.620, p = 0.060, np2 = 0.040. No other phenotypes were significantly related to impulsivity scores (Supplementary Material A).
Impulsivity and EEG
When cortical activity markers were correlated to impulsivity scores, iAPF was positively correlated to both emotion-related impulsivity factors, | r's |> 0.246, p's < 0.050 (Fig. 3). No other significant results for cortical activity makers with impulsivity were detected. Nevertheless, as shown in Fig. 3, non-significant trends between three binary prefrontal asymmetry LCs (Fp2/Fp1, F2/F1, F4/F3) were observed, 0.113 > p's > 0.092. Age was not correlated with any EEG markers.
Pearson’s correlation coefficients of cortical activity markers and impulsivity scores (T0). PIF, Pervasive Influence of Feelings; LFT, (Lack of) Follow-Through; FTA, Feelings Trigger Action; LC, Laterality Coefficient; iAPF, individual alpha peak frequency. Significance for the asymmetry: #p < 0.008. For the rest: *p < 0.050; **p < 0.010; ***p < 0.001
Multiple Linear Regression Models
Multiple linear regression models are reported in Table 1 for Feelings Trigger Action at screening (n = 90), and for a smaller sample, all three impulsivity scales at T0 (n = 67). Of the four models, only the two with Feelings Trigger Action were significant. Model 1 explained 21.9% (adj. R2 = 15.2%) of the total variance of Feelings Trigger Action at screening (p < 0.010). Out of the five predictors included in the Feelings Trigger Action screening model, only 5-HTTLPR and MAO-A polymorphisms were significant. Beta scores (Table 1) show that carriers of 5-HTTLPRHigh and MAO-AHigh phenotypes have lower Feelings Trigger Action scores when compared to the low transcriptional activity phenotypes. When standardized, the 5-HTTLPR beta scores had the strongest magnitude (Table 1). When iAPF, F4/F3 LC, and their interaction terms with 5-HTTLPR were added, the second model explained 36.7% (adj. R2 = 21.2%) of the total variance (p < 0.025). Out of the nine predictors, 5-HTTLPRModerate , iAPF, and their interaction term were significant (Table 1). When compared to 5-HTTLPRLow phenotype, 5-HTTLPRModerate carriers had lower Feelings Trigger Action scores. On the contrary, Feelings Trigger Action scores conjointly increased with iAPF. Nevertheless, the interaction term 5-HTTLPRModerate / iAPF had a negative beta score, indicating an inverse relation. Even though not significant, the interaction term 5-HTTLPRHigh / iAPF also had a negative beta score suggesting that the positive relation between iAPF and Feeling Trigger Action scores was very likely to be driven by 5-HTTLPRLow phenotypic category. Even though models 3 and 4 explained an important part of variance (respectively, 19.3% and 25.4%), neither was significant (Table 1).
Significant predictors of model 2 are displayed in Fig. 4. The graphic clearly illustrates the interaction between iAPF and 5-HTTLPR phenotypes, showing a strong positive link between iAPF and Feelings Trigger Action scores in participants with the 5-HTTLPRLow phenotype.
Discussion
Impulsivity triggered by positive or negative emotions has been shown to be more clinically relevant than other forms unrelated to emotions [73, 74]. Given the evidence that impulsivity is linked not only to serotonergic neurotransmission polymorphisms (i.e., 5-HTTLPR, STin2, and MAO-A) but also to cortical activity (i.e., iAPF and prefrontal alpha asymmetry), this explorative study aimed to assess the conjoint importance of these variables in explaining emotion-related impulsivity. The study examined two forms of emotion-related impulsivity— Feelings Trigger Action, which captures tendencies to engage in regrettable behavior in response to emotion, and Pervasive Influence of Feelings, which covers unconstrained cognitive/motivational responses to emotions. Both forms of emotion-related impulsivity were linked to higher iAPF. Carriers of MAO-ALow and 5-HTTLPRLow phenotypes had higher Feelings Trigger Action scores. Furthermore, findings of the multiple regression models show that the 5-HTTLPR polymorphism, iAPF, and their interaction term are significant predictors of Feelings Trigger Action scores. Significant effects of the 5-HTTLPR polymorphism were observed for both administrations of the Feelings Trigger Action subscale. These findings support the endophenotypic approach to emotion-related impulsivity.
In line with previous research [9], the moderate to large effect sizes detected between Feelings Trigger Action scores and 5-HTTLPR (at screening and T0) and MAO-A (T0) transcriptional activity phenotypes confirm the importance of these polymorphisms in explaining emotion-related impulsivity levels. This fits with the findings from several studies showing that carriers of 5-HTTLPRLow and MAO-ALow phenotypes show hyper-responsivity of the amygdala and anterior cingulate cortex during the display of negative emotional stimuli [75,76,77,78]. Even though it would be logical to relate the transcriptional activity levels of these genes to monoamine rates within neurons and synapses, research suggests that this relation is more complex. This reactivity is tied to decreased functional connectivity between the amygdala and anterior cingulate cortex in the 5-HTTLPRLow , compared with 5-HTTLPRHigh phenotype carriers [76]. The source of this functional connectivity difference might arise during fetal neurodevelopment, in that 5-HTTLPRLow and MAO-ALow phenotypes have, respectively, fewer 5-HTT and MAO-A mRNA levels in placenta tissue [56]. Therefore, emotion-related impulsivity levels could be driven by fetal monoamine levels affecting structural connectivity, and consequently functional interactions within neural circuits that regulate emotional reactivity.
We observed only non-significant trend-level correlations between prefrontal alpha asymmetry and emotion-related impulsivity. Prefrontal alpha asymmetry, though, is influenced not just by trait-like characteristics but also tends to shift in a state-dependent manner, and our current study design did not allow us to consider the state-like dynamic variation. We also did not record handedness preference or state levels of affect and motivation, which can potentially influence asymmetry results. Finally, post hoc power analyses (power > 0.8) suggest that effect sizes below r = 0.260 were probably undetectable.
Despite the null effect in the correlational analyses for alpha asymmetry, our findings suggest that a form of emotion-related impulsivity related to regrettable behavior (Feelings Trigger Action) is tied to a set of neural (iAPF) and genetic variables (5-HTTLPR and MAO-A). Intriguingly, results were not generalized to unconstrained cognitive or motivational responses to emotion (Pervasive Influence of Feelings), dovetailing with previous work to suggest the importance of distinguishing between these two factors in understanding neurocognitive correlates and psychopathology.
Univariate analyses indicated that the cognitive and behavioral forms of emotion-related impulsivity were both correlated to higher iAPF. These results are counterintuitive when considering research linking decreasing iAPF to central system pathologies [50] and high resting iAPF to high executive function performances [79]. Nonetheless, it is important to recall that these measurements were obtained from healthy participants without any emotional trigger. Furthermore, regression model 2 (Table 1) did put in evidence a significant interaction between the two predictors (i.e., iAPF and 5-HTTLPR), displaying a very different relationship between iAPF and Feelings Trigger Action scores per 5-HTTLPR phenotypes (Fig. 4). The positive relation depicted in the univariate analysis and in the beta score of iAPF (Table 1, model 2) appeared to be driven by the large effect in the carriers of the 5-HTTPLRLow (n = 19, R2 = 0.330, p < 0.010). These ties were of negligible magnitude for 5-HTTLPRModerate (n = 30, R2 = 0.001, p = 0.900) and 5-HTTLPRHigh (n = 11, R2 = 0.014, p = 0.726). More research investigating the link between 5-HTTLPR and iAPF is warranted. Moreover, 5-HTTLPR should now be considered systematically in iAPF research to have a better understanding of the results.
Despite the intriguing and novel findings, the results of this study should be interpreted within the context of several limitations. First, even though this study used a multifactorial endophenotypic approach, the scope of the investigation was limited. For example, polymorphisms in the dopaminergic pathway [80, 81], circulating levels of tryptophan [53, 82], central serotonin shortage [83, 84], and activity of the kynurenine pathway [53] have been tied to impulsivity but were not assessed in the present study. Second, the recruited sample had only a modest range of impulsivity scores (the 35% highest emotion-related impulsivity scores), which may have reduced result variance and constrained effect sizes. Third, even though most research in the field of alpha asymmetry underlying personality traits recommend resting EEG (e.g., [31, 32, 39, 63]), another body of research suggests that this condition might not be the most optimal [65, 85]. They argue that uncontrolled experimental conditions may affect resting measures and that individuals can engage in a variety of mental states that are not controlled during the resting tasks. As we used resting EEG condition, we cannot exclude that these parameters may have affected our results. Including an additional motivational induction paradigm followed by EEG measures [65, 85] in future studies may provide further insights into the relationship between frontal asymmetry and impulsivity. Finally, it is worth noting that future studies might also consider using clinical interviews and behavioral tests (e.g., Go/No-Go test) to gain deeper insights into impulsivity.
In conclusion, our results highlight the importance of using endophenotypic approaches to characterize impulsivity, demonstrating that the 5-HTTLPR polymorphism, iAPF, and their interaction are relevant predictors of a key form of emotion-related impulsivity involving regrettable behavior. Moreover, carriers of 5-HTTPLRLow and MAO-ALow phenotypes showed higher levels of this form of emotion-related impulsivity than did those with other phenotypes. This multifactorial neurogenetic approach to impulsivity could be applied to developing better identification and prediction for impulsivity-related disorders. Our findings were specific to emotion-related impulsivity involving regrettable behavior, which is of importance given the burgeoning literature suggesting that this form of impulsivity is uniquely powerful in predicting externalizing, suicidal behavior, and other key outcomes [1]. Evaluating conjoint changes between impulsivity and epigenetic mechanisms in serotonergic neurotransmission polymorphisms (e.g., DNA methylation, histone acetylation) represents another rich domain for future work that can be incorporated into endophenotypic approaches.
Availability of Data and Materials
Data used in this analysis are provided in Supplementary Material B.
Code Availability
The R code used to perform this analysis is provided in Supplementary Material C.
References
Johnson SL, Tharp JA, Peckham AD et al (2017) A path model of different forms of impulsivity with externalizing and internalizing psychopathology: towards greater specificity. Br J Clin Psychol 56:235–252. https://doi.org/10.1111/bjc.12135
Carver CS, Johnson SL, Joormann J (2008) Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol Bull 134:912–943. https://doi.org/10.1037/a0013740
Swann AC, Anderson JC, Dougherty DM, Moeller FG (2001) Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Res 101:195–197. https://doi.org/10.1016/S0165-1781(00)00249-3
Guerrero MD, Barnes JD, Walsh JJ et al (2019) 24-hour movement behaviors and impulsivity. Pediatrics 144:e20190187. https://doi.org/10.1542/peds.2019-0187
Corruble E, Damy C, Guelfi JD (1999) Impulsivity: a relevant dimension in depression regarding suicide attempts? J Affect Disord 53:211–215. https://doi.org/10.1016/s0165-0327(98)00130-x
Barratt E (1965) Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychol Rep 16:547–554. https://doi.org/10.2466/pr0.1965.16.2.547
Dickman SJ (1990) Functional and dysfunctional impulsivity: personality and cognitive correlates. J Pers Soc Psychol 58:95–102. https://doi.org/10.1037/0022-3514.58.1.95
Whiteside SP, Lynam DR (2001) The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif 30:669–689. https://doi.org/10.1016/S0191-8869(00)00064-7
Carver CS, Johnson SL, Joormann J et al (2011) Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychol Sci 22:589–595. https://doi.org/10.1177/0956797611404085
Carver CS, Johnson SL, Kim Y (2016) Mu opioid receptor polymorphism, early social adversity, and social traits. Soc Neurosci 11:515–524. https://doi.org/10.1080/17470919.2015.1114965
Smith GT, Cyders MA (2016) Integrating affect and impulsivity: the role of positive and negative urgency in substance use risk. Drug Alcohol Depend 163:3–12. https://doi.org/10.1016/j.drugalcdep.2015.08.038
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260. https://doi.org/10.1016/j.pbb.2007.12.021
Tricklebank MD, Daly E (2018) The serotonin system: history, neuropharmacology, and pathology, 1st Ed. London Academic Press (Elsevier)
Canli T, Lesch KP (2007) Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10:1103–1109. https://doi.org/10.1038/nn1964
Williams LM, Gatt JM, Kuan SA et al (2009) A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology 34:1797–1809. https://doi.org/10.1038/npp.2009.1
Iurescia S, Seripa D, Rinaldi M (2017) Looking beyond the 5-HTTLPR polymorphism: genetic and epigenetic layers of regulation affecting the serotonin transporter gene expression. Mol Neurobiol 54:8386–8403. https://doi.org/10.1007/s12035-016-0304-6
Niesler B, Kapeller J, Fell C et al (2010) 5-HTTLPR and STin2 polymorphisms in the serotonin transporter gene and irritable bowel syndrome: effect of bowel habit and sex. Eur J Gastroenterol Hepatol 22:856–861. https://doi.org/10.1097/MEG.0b013e32832e9d6b
Kraft JB, Slager SL, McGrath PJ, Hamilton SP (2005) Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry 58:374–381. https://doi.org/10.1016/j.biopsych.2005.04.048
Lesch K-P, Bengel D, Heils A et al (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 42:2482–2488. https://doi.org/10.1126/science.274.5292.1527
Oades RD, Lasky-Su J, Christiansen H et al (2008) The influence of serotonin-and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): findings from a family-based association test (FBAT) analysis. Behav Brain Funct 4:1–14. https://doi.org/10.1186/1744-9081-4-48
Evans J, Battersby S, Ogilvie AD et al (1997) Association of short alleles of a VNTR of the serotonin transporter gene with anxiety symptoms in patients presenting after deliberate self harm. Neuropharmacology 36:439–443. https://doi.org/10.1016/S0028-3908(97)00027-0
Ogilvie AD, Battersby S, Bubb VJ et al (1996) Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet 347:731–733. https://doi.org/10.1016/S0140-6736(96)90079-3
Bellivier F, Leroux M, Henry C et al (2002) Serotonin transporter gene polymorphism influences age at onset in patients with bipolar affective disorder. Neurosci Lett 334:17–20. https://doi.org/10.1016/S0304-3940(02)01029-7
De Lara CL, Dumais A, Rouleau G et al (2006) STin2 variant and family history of suicide as significant predictors of suicide completion in major depression. Biol Psychiatry 59:114–120. https://doi.org/10.1016/j.biopsych.2005.06.021
Rodríguez-Ramos Á, Antonio Moriana J, García-Torres F et al (2019) Emotional stability is associated with the MAOA promoter uVNTR polymorphism in women. Brain Behav 9:e01376. https://doi.org/10.1002/brb3.1376
Brunner HG, Nelen M, Breakefield XO et al (1993) Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578–580. https://doi.org/10.1126/science.8211186
Furlong RA, Ho L, Rubinsztein JS et al (1999) Analysis of the monoamine oxidase A (MAOA) gene in bipolar affective disorder by association studies, meta-analyses, and sequencing of the promoter. Am J Med Genet Neuropsychiatr Genet 88:398–406. https://doi.org/10.1002/(SICI)1096-8628(19990820)88:4%3c398::AID-AJMG18%3e3.0.CO;2-Y
Stetler DA, Davis C, Leavitt K et al (2014) Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. J Psychiatr Res 58:69–75. https://doi.org/10.1016/j.jpsychires.2014.07.006
Papousek I, Reiser EM, Schulter G et al (2013) Serotonin transporter genotype (5-HTTLPR) and electrocortical responses indicating the sensitivity to negative emotional cues. Emotion 13:1173–1181. https://doi.org/10.1037/a0033997
Rosadini G, Rossi GF (1967) On the suggested cerebral dominance for consciousness. Brain 90:101–112. https://doi.org/10.1093/brain/90.1.101
Gable PA, Neal LB, Threadgill AH (2018) Regulatory behavior and frontal activity: considering the role of revised-BIS in relative right frontal asymmetry. Psychophysiology 55:1–18. https://doi.org/10.1111/psyp.12910
Sutton SK, Davidson RJ (1997) Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci 8:204–210. https://doi.org/10.1111/j.1467-9280.1997.tb00413.x
Tomarken AJ, Davidson RJ (1994) Frontal brain activation in repressors and nonrepressors. J Abnorm Psychol 103:339–349. https://doi.org/10.1037/0021-843X.103.2.339
Cyders MA, Zapolski TCB, Combs JL et al (2010) Experimental effect of positive urgency on negative outcomes from risk taking and on increased alcohol consumption. Psychol Addict Behav 24:367–375. https://doi.org/10.1037/A0019494
Zapolski TCB, Cyders MA, Smith GT (2009) Positive urgency predicts illegal drug use and risky sexual behavior. Psychol Addict Behav 23:348–354. https://doi.org/10.1037/A0014684
Pfurtscheller G, Stancák A, Neuper C (1996) Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol 24:39–46. https://doi.org/10.1016/S0167-8760(96)00066-9
Harmon-Jones E, Gable PA, Peterson CK (2010) The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol 84:451–462. https://doi.org/10.1016/j.biopsycho.2009.08.010
Neal LB, Gable PA (2017) Regulatory control and impulsivity relate to resting frontal activity. Soc Cogn Affect Neurosci 12:1377–1383. https://doi.org/10.1093/scan/nsx080
Gable PA, Mechin NC, Hicks JA, Adams DL (2015) Supervisory control system and frontal asymmetry: neurophysiological traits of emotion-based impulsivity. Soc Cogn Affect Neurosci 10:1310–1315. https://doi.org/10.1093/scan/nsv017
Muhlert N, Lawrence AD (2015) Brain structure correlates of emotion-based rash impulsivity. Neuroimage 115:138–146. https://doi.org/10.1016/j.neuroimage.2015.04.061
Davidson RJ (2000) Affective style, psychopathology, and resilience: brain mechanisms and plasticity. Am Psychol 55:1196–1214. https://doi.org/10.1037/0003-066X.55.11.1196
Davidson RJ (2002) Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51:68–80. https://doi.org/10.1016/S0006-3223(01)01328-2
Jackson DC, Malmstadt JR, Larson CL, Davidson RJ (2000) Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 37:515–522. https://doi.org/10.1111/1469-8986.3740515
Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE (2002) Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229. https://doi.org/10.1162/089892902760807212
Anokhin AP, Van Baal GCM, Van Beijsterveldt CEM et al (2001) Genetic correlation between the P300 event-related brain potential and the EEG power spectrum. Behav Genet 31:545–554. https://doi.org/10.1023/A:1013341310865
Anokhin AP, Müller V, Lindenberger U et al (2006) Genetic influences on dynamic complexity of brain oscillations. Neurosci Lett 397:93–98. https://doi.org/10.1016/j.neulet.2005.12.025
Smit CM, Wright MJ, Hansell NK et al (2005) Genetic variation of individual alpha frequency (IAF) and alpha power in a large adolescent twin sample. Int J Psychophysiol 61:235–243. https://doi.org/10.1016/j.ijpsycho.2005.10.004
Gasser T, Bächer P, Steinberg H (1985) Test-retest reliability of spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol 60:312–319. https://doi.org/10.1016/0013-4694(85)90005-7
Salinsky MC, Oken BS, Morehead L (1991) Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol 79:382–392. https://doi.org/10.1016/0013-4694(91)90203-G
Grandy TH, Werkle-Bergner M, Chicherio C et al (2013) Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 50:570–582. https://doi.org/10.1111/psyp.12043
Knyazev GG, Levin EA, Savostyanov AN (2008) Impulsivity, anxiety, and individual differences in evoked and induced brain oscillations. Int J Psychophysiol 68:242–254. https://doi.org/10.1016/j.ijpsycho.2008.02.010
Van Beijsterveldt CEM, Van Baal GCM (2002) Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol 61:111–138. https://doi.org/10.1016/S0301-0511(02)00055-8
Javelle F, Bloch W, Knoop A et al (2021) Toward a neuroprotective shift: eight weeks of high intensity interval training reduces the neurotoxic kynurenine activity concurrently to impulsivity in emotionally impulsive humans—a randomized controlled trial. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2021.04.020
Davies A, Rodriguez-Vicente AE, Austin G et al (2020) Serotonin re-uptake transporter gene polymorphisms are associated with imatinib-induced diarrhoea in chronic myeloid leukaemia patients - Supplementary material. Sci Rep 53:1689–1699. https://doi.org/10.1017/CBO9781107415324.004
Sabol SZ, Hu S, Hamer D (1998) A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103:273–279. https://doi.org/10.1007/s004390050816
Zhang H, Smith GN, Liu X, Holden JJ (2010) Association of MAOA, 5-HTT, and NET promoter polymorphisms with gene expression and protein activity in human placentas. Physiol Genomics 42:85–92. https://doi.org/10.1152/physiolgenomics.00220.2009
Auerbach RP, Stewart JG, Johnson SL (2017) Impulsivity and suicidality in adolescent inpatients. J Abnorm Child Psychol 45:91–103. https://doi.org/10.1007/s10802-016-0146-8
Carver CS, La VL, Kuhl J, Ganellen RJ (1988) Cognitive concomitants of depression: a further examination of the roles of generalization, high standards, and self-criticism. J Soc Clin Psychol 7:350–365. https://doi.org/10.1521/jscp.1988.7.4.350
Cyders MA, Smith GT, Spillane NS et al (2007) Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol Assess 19:107–118. https://doi.org/10.1037/1040-3590.19.1.107
Javelle F, Wiegand M, Joormann J et al (2020) The German Three Factor Impulsivity Index: confirmatory factor analysis and ties to demographic and health-related variables. Pers Individ Dif 171:1–12. https://doi.org/10.1016/j.paid.2020.110470
Gramfort A (2013) MEG and EEG data analysis with MNE-Python. Front Neurosci 7:267. https://doi.org/10.3389/fnins.2013.00267
Bigdely-Shamlo N, Mullen T, Kothe C et al (2015) The PREP pipeline: standardized preprocessing for large-scale EEG analysis. Front Neuroinform 9:1–19. https://doi.org/10.3389/fninf.2015.00016
Harmon-Jones E, Allen JJB (1997) Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol 106:159–163. https://doi.org/10.1037/0021-843X.106.1.159
Porac C, Coren S (1981) Lateral preferences and human behavior. Lateral Prefer Hum Behav. https://doi.org/10.1007/978-1-4613-8139-6
Coan JA, Allen JJB, McKnight PE (2006) A capability model of individual differences in frontal EEG asymmetry. Biol Psychol 72:198. https://doi.org/10.1016/J.BIOPSYCHO.2005.10.003
Papousek I, Schulter G (2002) Covariations of EEG asymmetries and emotional states indicate that activity at frontopolar locations is particularly affected by state factors. Psychophysiology 39:350–360. https://doi.org/10.1017/S0048577201393083
Pivik RT, Broughton RJ, Coppola R et al (1993) Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology 30:547–558. https://doi.org/10.1111/J.1469-8986.1993.TB02081.X
Field A, Miles J, Field Z (2013) Discovering statistics using R, 4th Ed. SAGE Publications Ltd, Los Angeles | London | New Delhi | Singapore | Washington DC
Eysenck SBG, Pearson PR, Easting G, Allsopp JF (1985) Age norms for impulsiveness, venturesomeness and empathy in adults. Pers Individ Dif 6:613–619. https://doi.org/10.1016/0191-8869(85)90011-X
Madley-Dowd P, Hughes R, Tilling K, Heron J (2019) The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 110:63–73. https://doi.org/10.1016/J.JCLINEPI.2019.02.016
Dong Y, Peng CYJ (2013) Principled missing data methods for researchers. SpringerPlus 2:1–17. https://doi.org/10.1186/2193-1801-2-222/TABLES/3
Bowerman BL, O'Connell RT (1990) Linear statistical models : an applied approach, 2nd Ed. PWS-Kent Pub. Co, Boston
Carver CS, Johnson SL, Timpano KR (2017) Toward a functional view of the p factor in psychopathology. Clin Psychol Sci 5:880–889. https://doi.org/10.1177/2167702617710037
Peters JR, Eisenlohr-Moul TA, Walsh EC, Derefinko KJ (2019) Exploring the pathophysiology of emotion-based impulsivity: the roles of the sympathetic nervous system and hostile reactivity. Psychiatry Res 267:368–375. https://doi.org/10.1016/j.psychres.2018.06.013
Hariri AR, Mattay VS, Tessitore A et al (2002) Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403. https://doi.org/10.1126/science.1071829
Pezawas L, Meyer-Lindenberg A, Drabant EM et al (2005) 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834. https://doi.org/10.1038/nn1463
Lee BT, Ham BJ (2008) Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. NeuroReport 19:515–519. https://doi.org/10.1097/WNR.0b013e3282f94294
Meyer-Lindenberg A, Buckholtz JW, Kolachana B et al (2006) Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A 103:6269–6274. https://doi.org/10.1073/pnas.0511311103
Haegens S, Cousijn H, Wallis G et al (2014) Inter- and intra-individual variability in alpha peak frequency. Neuroimage 92:46–55. https://doi.org/10.1016/j.neuroimage.2014.01.049
Congdon E, Canli T (2005) The endophenotype of impulsivity: reaching consilience through behavioral, genetic, and neuroimaging approaches. Behav Cogn Neurosci Rev 4:262–281. https://doi.org/10.1177/1534582305285980
Yates JR, Darna M, Gipson CD et al (2015) Dissociable roles of dopamine and serotonin transporter function in a rat model of negative urgency. Behav Brain Res 291:201–208. https://doi.org/10.1016/j.bbr.2015.05.023
Javelle F, Li D, Zimmer P, Johnson SL (2019) Dietary intake of tryptophan tied emotion-related impulsivity in humans. Int J Vitam Nutr Res:1–8.https://doi.org/10.1024/0300-9831/a000608
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361. https://doi.org/10.1007/PL00005481
Fairbanks LA, Melega WP, Jorgensen MJ et al (2001) Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology 24:370–378. https://doi.org/10.1016/S0893-133X(00)00211-6
Rodrigues J, Allen JJB, Müller M, Hewig J (2021) Methods matter: an examination of factors that moderate predictions of the capability model concerning the relationship of frontal asymmetry to trait measures. Biol Psychol 158:107993. https://doi.org/10.1016/J.BIOPSYCHO.2020.107993
Acknowledgements
The authors want to thank Annika Voß for her advice regarding the polymorphism analyses and Anke Vogel for help in the recruitment of participants.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.J. and P.Z. did the conceptualization of the study. F.J. did the project administration. F.J. did the blood analyses and the polymorphism analyses with the help of A.S. advice. F.J., A.L., and T.H. did the EEG analysis. F.J. did the data curation and the data analysis. F.J. wrote the manuscript and made the figures. T.H., A.L., A.S., T.J., S.J., W.B., and P.Z. reviewed the manuscript and suggested edits.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the university review board before data collection (No. 166/2018).
Consent to Participate
All participants provided their consent to participate in the study before data collection.
Consent for Publication
All participants provided their consent for publication of the study results before data collection.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Javelle, F., Löw, A., Bloch, W. et al. Unraveling the Contribution of Serotonergic Polymorphisms, Prefrontal Alpha Asymmetry, and Individual Alpha Peak Frequency to the Emotion-Related Impulsivity Endophenotype. Mol Neurobiol 59, 6062–6075 (2022). https://doi.org/10.1007/s12035-022-02957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02957-6