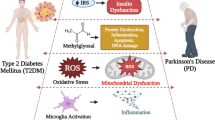
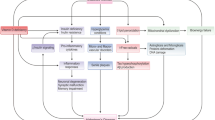
Abstract
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemic conditions. A higher risk of developing Parkinson’s disease (PD) in patients with T2DM has become evident in recent years. However, the molecular mechanisms underlying the interplay between T2DM and PD pathogenesis remain unknown. Nevertheless, emerging epidemiological studies have demonstrated many common molecular pathways that play an essential role in regulating normal cellular functioning are independently implicated in the progression and etiopathogenesis of T2DM and PD. This review summarizes some common shared pathophysiological mechanisms, including insulin resistance, inflammation, mitochondrial dysfunction, endoplasmic reticulum stress (ER stress), autophagy, and the ubiquitin–proteasome system (UPS) that independently mediate the onset and etiopathogenesis of T2DM and PD. In this review, we summarize the studies that have reported the relationship between T2DM and PD. This review will provide insights into the common involvement of molecular pathways that may provide alternative treatment strategies for both T2DM and PD.
Graphical abstract





Similar content being viewed by others
Data Availability
No data is used in this review.
Abbreviations
- 6-OHDA:
-
6 Hydroxy dopamine
- ABA:
-
Abscisic acid
- AIM2 inflammasome:
-
Absent in melanoma 2 inflammasome
- AKT:
-
Protein kinase B
- AMP:
-
Adenosine monophosphate
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ATF4:
-
Activating transcription factor 4
- ATF6:
-
Activating transcription factor 6
- Atg:
-
Autophagy related
- BBB:
-
Blood-brain barrier
- Bcl-2:
-
B cell lymphoma 2
- BDNF:
-
Brain-derived neurotrophic factor
- BiP:
-
Immunoglobulin heavy chain binding protein
- cAMP:
-
Cyclic AMP
- CAT:
-
Catalase
- CHOP:
-
C/EBP homologous protein
- CMA:
-
Chaperone-mediated autophagy
- COX-2:
-
Cyclooxygenase-2
- CREB:
-
CAMP response element binding protein
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopamine
- DA-ergic:
-
Dopaminergic
- DAMP:
-
Damaged-associated molecular pattern
- DJ-1:
-
DnaJ-1
- DM:
-
Diabetes mellitus
- Drp1:
-
Dynamin-related protein-1
- EIF2AK3:
-
Eukaryotic translation initiation factor 2 alpha kinase 3
- eIF2α:
-
Eukaryotic initiation factor 2 alpha
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated kinases ERK1
- ETC:
-
Electron transport chain
- Fis1:
-
Fission 1
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP-1:
-
Glucagon-like peptide–l
- GLP-1R:
-
GLP-1 receptor
- GLUT4:
-
Glucose transporters type 4
- GPCR:
-
G protein-coupled receptor
- Grp78 protein:
-
Glucose-regulated protein 78
- GSK3-β:
-
Glycogen synthase kinase-3beta
- hA:
-
Human amylin
- HFD:
-
High-fat diet
- h-IAPP:
-
Human islet amyloid polypeptide
- Hrd1:
-
HMG–CoA reductase degradation protein-1
- IAPP:
-
Islet amyloid polypeptide
- Iba-1:
-
Ionized calcium binding adaptor molecule 1
- IFN-γ:
-
Interferon gamma
- IkBα:
-
NF-kappa-B inhibitor alpha (I-kappa-B-alpha) (IkB-alpha)
- IKK-β:
-
Inhibitor of nuclear factor-kappaB (IkappaB) kinase beta
- IL:
-
Interleukin
- IL-18R α/β:
-
IL-18 receptor α/β
- iNOS:
-
Inducible nitric oxide synthase
- IPD:
-
Idiopathic PD
- IPL:
-
Inferior parietal lobule
- IRE1α:
-
Inositol requiring enzyme 1 α
- IRN:
-
Isorhynchophylline
- IRS:
-
Insulin receptor substrate
- LAMP1:
-
Lysosomal-associated membrane protein type 1
- LB:
-
Lewy body
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- LC:
-
Locus coeruleus
- L-DOPA:
-
Levodopa
- LRRK2:
-
Leucine-rich repeat kinase 2
- MafA:
-
Musculoaponeurotic fibrosarcoma oncogene family A
- MAPK:
-
Mitogen-activated protein kinase
- MFG:
-
Middle frontal gyrus
- MFN:
-
Mitochondrial mitofusin
- MHC:
-
Major histocompatibility complex
- Miro 1:
-
Mitochondrial rho GTPase 1
- MMP:
-
Mitochondrial membrane potential
- MPP+ :
-
1-Methyl-4-phenylpyridinium
- MPTP:
-
1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydrodropyridine
- MSK1:
-
Mitogen and stress-activated kinase-1
- mTOR:
-
Mechanistic target of rapamycin
- NADH:
-
Nicotinamide adenine dinucleotide (NAD) + hydrogen (H)
- NADPH-oxidase:
-
Nicotinamide adenine nucleotide phosphate oxidase
- NF-ΚB:
-
Nuclear factor-κB
- NLRP3 inflammasome:
-
NOD-, LRR-, and pyrin domain-containing 3 inflammasome
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- P2X4R:
-
P2X purinoceptor 4
- P2X7R:
-
P2X purinoceptor 7
- Pael-R:
-
Parkin-associated endothelin receptor-like receptor
- Park2:
-
Ub ligase Parkin
- PARP:
-
Poly ADP-ribose polymerase
- PD:
-
Parkinson’s disease
- PERK:
-
Pancreatic ER kinase (PKR)–like ER kinase
- PGC1-α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1
- PI3K:
-
Phosphatidylinositol 3-phosphate kinase
- PINK1:
-
PTEN-induced putative phosphatase 1
- PKA:
-
Protein kinase A
- PKB:
-
Protein kinase B
- PPARs:
-
Peroxisome proliferator-activated receptors
- PQ:
-
Paraquat
- PTEN:
-
Phosphatase and tensin homolog
- Rab10:
-
Ras-related protein Rab 10
- ROS:
-
Reactive oxygen species
- SNCA:
-
Synuclein alpha
- SNpc:
-
Substantia nigra pars compacta
- SOCE:
-
Store-operated calcium entry (SOCE)
- SOCS:
-
Suppressor of cytokine signaling
- SOD:
-
Superoxide dismutase
- STZ:
-
Streptozotocin
- SUMO-1:
-
Substrate for ubiquitin-like modifier-1
- T2DM:
-
Type 2 diabetes mellitus
- TFB1M:
-
Transcription factor B1 mitochondrial
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
- TOM20:
-
Translocase of the outer membrane 20
- TRPC1:
-
Transient receptor potential channel 1
- UCH-L1:
-
Ubiquitin carboxy-terminal hydrolase L1
- UCP2:
-
Uncoupling protein 2
- UPDRS:
-
Unified Parkinson Disease Rating Scale
- UPR:
-
Unfolded protein response
- UPS:
-
Ubiquitin proteasome system
- VDAC:
-
Voltage-dependent anion-selective channel
- WFS1:
-
Wolfram syndrome gene 1
- XBP1:
-
X-box-binding protein 1
References
Hong CT, Chen KY, Wang W, Chiu JY, Wu D, Chao TY, Hu CJ, Chau KD, Bamodu OA (2020) Insulin resistance promotes Parkinson’s disease through aberrant expression of α-synuclein, mitochondrial dysfunction, and deregulation of the polo-like kinase 2 signaling. Cells 9(3):740. https://doi.org/10.3390/cells9030740
Xuan WT, Wang H, Zhou P, Ye T, Gao HW, Ye S, Wang JH, Chen ML, Song H, Wang Y, Cai B (2020) Berberine ameliorates rats model of combined s disease and type diabetes mellitus via the suppression of endoplasmic reticulum stress. Biotech 10(8):359. https://doi.org/10.1007/s13205-020-02354-7
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3:17013
Yang YW, Hsieh TF, Li CI, Liu CS, Lin WY, Chiang JH, Li TC, Lin CC (2017) Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 96(3):e5921. https://doi.org/10.1097/MD.0000000000005921
Cereda E, Barichella M, Pedrolli C, Klersy C, Cassani E, Caccialanza R, Pezzoli G (2011) Diabetes and risk of Parkinson’s disease: a systematic review and meta-analysis. Diabetes Care 34(12):2614–2623. https://doi.org/10.2337/dc11-1584
Santiago JA, Potashkin JA (2013) Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med 19(3):176–186. https://doi.org/10.1016/j.molmed.2013.01.002
Katila N, Bhurtel S, Shadfar S, Srivastav S, Neupane S, Ojha U, Jeong GS, Choi DY (2017) Metformin lowers α-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 125:396–407. https://doi.org/10.1016/j.neuropharm.2017.08.015
Grieco M, Giorgi A, Gentile MC, d’Erme M, Morano S, Maras B, Filardi T (2019) Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci 18(13):1112. https://doi.org/10.3389/fnins.2019.01112
Sandyk R (1993) The relationship between diabetes mellitus and Parkinson’s disease. Int J Neurosci 69(1–4):125–130. https://doi.org/10.3109/00207459309003322
Pagano G, Polychronis S, Wilson H, Giordano B, Ferrara N, Niccolini F, Politis M (2018) Diabetes mellitus and Parkinson disease. Neurology 8 90(19):1654–1662. https://doi.org/10.1212/WNL.0000000000005475
XuQ PY, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H (2011) Diabetes and risk of Parkinson’s disease. Diabetes Care 34(4):910–915. https://doi.org/10.2337/dc10-1922
Qi L, Cornelis MC, Kraft P, Stanya KJ, Linda Kao WH, Pankow JS, Dupuis J, Florez JC, Fox CS, Paré G, Sun Q, Girman CJ, Laurie CC, Mirel DB, Manolio TA, Chasman DI, Boerwinkle E, Ridker PM, Hunter DJ, Meigs JB, Lee CH, Hu FB, van Dam RM (2010). Meta-Analysis of Glucose and Insulin-related traits Consortium (MAGIC); Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium. Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet 19 (13):2706–15 https://doi.org/10.1093/hmg/ddq156
Xiromerisiou G, Hadjigeorgiou GM, Papadimitriou A, Katsarogiannis E, Gourbali V, Singleton AB (2008) Association between AKT1 gene and Parkinson’s disease a protective haplotype. Neurosci Lett 436(2):232–4. https://doi.org/10.1016/j.neulet.2008.03.026
Jain D, Jain R, Eberhard D, Eglinger J, Bugliani M, Piemonti L, Marchetti P, Lammert E (2012) Age- and diet-dependent requirement of DJ-1 for glucose homeostasis in mice with implications for human type 2 diabetes. J Mol Cell Biol 4(4):221–230. https://doi.org/10.1093/jmcb/mjs025
Fukushima T, Tan X, Luo Y, Kanda H (2011) Serum vitamins and heavy metals in blood and urine, and the correlations among them in Parkinson’s disease patients in China. Neuroepidemiology 36(4):240–244. https://doi.org/10.1159/000328253
Yue X, Li H, Yan H, Zhang P, Chang L, Li T (2016) Risk of Parkinson disease in diabetes mellitus: an updated meta-analysis of population-based cohort studies. Medicine (Baltimore) 95(18):e3549. https://doi.org/10.1097/MD.0000000000003549
Santos García D, Suárez Castro E, Expósito I, de Deus T, Tuñas C, Aneiros A, López Fernández M, Núñez Arias D, Bermúdez Torres M (2017) Comorbid conditions associated with Parkinson’s disease: a longitudinal and comparative study with Alzheimer disease and control subjects. J Neurol Sci 15(373):210–215. https://doi.org/10.1016/j.jns.2016.12.046
Cheong JLY, de Pablo-Fernandez E, Foltynie T, Noyce AJ (2020) The association between type 2 diabetes mellitus and Parkinson’s disease. J Parkinsons Dis 10(3):775–789. https://doi.org/10.3233/JPD-191900
Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G (2012) Clinical features of Parkinson disease when onset of diabetes came first a case control study. Neurology 78(19):1507–11. https://doi.org/10.1212/WNL.0b013e3182553cc9
Bohnen NI, Kotagal V, Müller ML, Koeppe RA, Scott PJ, Albin RL, Frey KA, Petrou M (2014) Diabetes mellitus is independently associated with more severe cognitive impairment in Parkinson disease. Parkinsonism Relat Disord 20(12):1394–8. https://doi.org/10.1016/j.parkreldis.2014.10.008
Hölscher C (2020) Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs 29(4):333–348. https://doi.org/10.1080/13543784.2020.1738383
Sirangelo I, Borriello M, Vilasi S, Iannuzzi C (2020) Hydroxytyrosol inhibits protein oligomerization and amyloid aggregation in human insulin. Int J Mol Sci 21(13):4636. https://doi.org/10.3390/ijms21134636
Cho JH, Kim JW, Shin JA, Shin J, Yoon KH (2011) β-cell mass in people with type 2 diabetes. J Diabetes Investig 2(1):6–17. https://doi.org/10.1111/j.2040-1124.2010.00072.x
Love KM, Liu J, Regensteiner JG, Reusch JEB, Liu Z (2020) GLP-1 and insulin regulation of skeletal and cardiac muscle microvascular perfusion in type 2 diabetes. J Diabetes 12(7):488–498. https://doi.org/10.1111/1753-0407.13045
Mukherjee A, Morales-Scheihing D, Butler PC, Soto C (2015) Type 2 diabetes as a protein misfolding disease. Trends Mol Med 21(7):439–449. https://doi.org/10.1016/j.molmed.2015.04.005
Bosco D, Plastino M, Cristiano D, Colica C, Ermio C, De Bartolo M, Mungari P, Fonte G, Consoli D, Consoli A, Fava A (2012) Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci 315(1–2):39–43. https://doi.org/10.1016/j.jns.2011.12.008
Ahmad MH, Fatima M, Mondal AC (2019) Role of hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis and insulin signaling in the pathophysiology of Alzheimer’s disease. Neuropsychobiology 77(4):197–205. https://doi.org/10.1159/000495521
Hong CT, Chen KY, Wang W, Chiu JY, Wu D, Chao TY, Hu CJ, Chau KD, Bamodu OA (2020) Insulin resistance promotes Parkinson’s disease through aberrant expression of α-synuclein, mitochondrial dysfunction, and deregulation of the polo-like kinase 2 signaling. Cells 9(3):740. https://doi.org/10.3390/cells9030740
Craft S, Watson GS (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3(3):169–78. https://doi.org/10.1016/S1474-4422(04)00681-7
Sekar S, Taghibiglou C (2018) Elevated nuclear phosphatase and tensin homolog (PTEN) and altered insulin signaling in substantia nigral region of patients with Parkinson’s disease. Neurosci Lett 666:139–143. https://doi.org/10.1016/j.neulet.2017.12.049
Athauda D, Foltynie T (2016) Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol 145–146:98–120. https://doi.org/10.1016/j.pneurobio.2016.10.001
Funk N, Munz M, Ott T, Brockmann K, Wenninger-Weinzierl A, Kühn R, Vogt-Weisenhorn D, Giesert F, Wurst W, Gasser T, Biskup S (2019) The Parkinson’s disease-linked leucine-rich repeat kinase 2 (LRRK2) is required for insulin-stimulated translocation of GLUT4. Sci Rep 9(1):4515. https://doi.org/10.1038/s41598-019-40808-y
Wang T, Yuan F, Chen Z, Zhu S, Chang Z, Yang W, Deng B, Que R, Cao P, Chao Y, Chan L, Pan Y, Wang Y, Xu L, Lyu Q, Chan P, Yenari MA, Tan EK, Wang Q. Vascular, inflammatory and metabolic risk factors in relation to dementia in Parkinson’s disease patients with type 2 diabetes mellitus. Aging (Albany NY) 12(15):15682–15704. https://doi.org/10.18632/aging.103776.
Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Yuan Q, Yu H, Xu W, Xie X (2019) New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol 20:247–260. https://doi.org/10.1016/j.redox.2018.09.025
Ahmad MH, Rizvi MA, Fatima M, Mondal AC (2021) Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol Cell Endocrinol 520:111093. https://doi.org/10.1016/j.mce.2020.111093
Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP (2013) Diabetes mellitus and inflammation. Curr Diab Rep 13(3):435–444. https://doi.org/10.1007/s11892-013-0375-y
Subba R, Sandhir R, Singh SP, Mallick BN, Mondal AC (2021) Pathophysiology linking depression and type 2 diabetes: Psychotherapy, physical exercise, and fecal microbiome transplantation as damage control. Eur J Neurosci 53(8):2870–2900. https://doi.org/10.1111/ejn.15136
Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH (2017) A new treatment strategy for Parkinson’s disease through the gut-brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant 26(9):1560–1571. https://doi.org/10.1177/0963689717721234
Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, Karmali M (2010) Type 2 diabetes mellitus and inflammation: prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes 3:173–186. https://doi.org/10.2147/dmsott.s9089
Conti P, Ronconi G, Kritas SK, Caraffa A, Theoharides TC (2018) Activated mast cells mediate low-grade inflammation in type 2 diabetes: interleukin-37 could be beneficial. Can J Diabetes 42(5):568–573. https://doi.org/10.1016/j.jcjd.2018.01.008
Cheng KT, Ong HL, Liu X, Ambudkar IS (2013) Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr 71:149–179. https://doi.org/10.1016/B978-0-12-407870-3.00007-X.PMID:23890115;
Wang J, Li Y, Lai K, Zhong Q, Demin KA, Kalueff AV, Song C (2020) High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: the role of peripheral and central inflammation, microglia and apoptosis. Prog Neuropsychopharmacol Biol Psychiatry 96:109752. https://doi.org/10.1016/j.pnpbp.2019.109752
Wang D, Wang H, Gao H, Zhang H, Zhang H, Wang Q, Sun Z (2011) P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci 10:28. https://doi.org/10.1186/s13578-020-00388-1
Sun X, Han F, Yi J, Han L, Wang B (2011) Effect of aspirin on the expression of hepatocyte NF-κB and serum TNF-α in streptozotocin-induced type 2 diabetic rats. J Korean Med Sci. 26(6):765–70. https://doi.org/10.3346/jkms.2011.26.6.765
Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI (2001) Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108(3):437–446. https://doi.org/10.1172/JCI11559
Pajares M, Rojo IA, Manda G, Boscá L, Cuadrado A (2020) Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells 9(7):1687. https://doi.org/10.3390/cells9071687
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4):354–365. https://doi.org/10.1016/j.nurt.2010.05.014
Chatterjee K, Roy A, Banerjee R, Choudhury S, Mondal B, Halder S, Basu P, Shubham S, Dey S, Kumar H (2020) Inflammasome and α-synuclein in Parkinson’s disease: a cross-sectional study. J Neuroimmunol 338:577089. https://doi.org/10.1016/j.jneuroim.2019.577089
Philips T, Robberecht W (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 10(3):253–263. https://doi.org/10.1016/S1474-4422(11)70015-1
Rodríguez-Cruz A, Romo-Mancillas A, Mendiola-Precoma J, Escobar-Cabrera JE, García-Alcocer G, Berumen LC (2019) Effect of valerenic acid on neuroinflammation in a MPTP-induced mouse model of Parkinson’s disease. IBRO Rep 8:28–35. https://doi.org/10.1016/j.ibror.2019.12.002
Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A (2008) Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol 167(1):90–95. https://doi.org/10.1093/aje/kwm260
Lee S, Lee Y, Ha S, Chung HY, Kim H, Hur JS, Lee J (2020) Anti-inflammatory effects of usnic acid in an MPTP-induced mouse model of Parkinson’s disease. Brain Res 1730:146642. https://doi.org/10.1016/j.brainres.2019.146642
O’Neill E, Yssel JD, McNamara C, Harkin A (2020) Pharmacological targeting of β2-adrenoceptors is neuroprotective in the LPS inflammatory rat model of Parkinson’s disease. Br J Pharmacol 177(2):282–297. https://doi.org/10.1111/bph.14862
Las G, Oliveira MF, Shirihai OS (2020) Emerging roles of β-cell mitochondria in type-2-diabetes. Mol Aspects Med 71:100843. https://doi.org/10.1016/j.mam.2019.100843
Kajihara N, Kukidome D, Sada K, Motoshima H, Furukawa N, Matsumura T, Nishikawa T, Araki E (2017) Low glucose induces mitochondrial reactive oxygen species via fatty acid oxidation in bovine aortic endothelial cells. J Diabetes Investig 8(6):750–761. https://doi.org/10.1111/jdi.12678
Sharoyko VV, Abels M, Sun J, Nicholas LM, Mollet IG, Stamenkovic JA, Göhring I, Malmgren S, Storm P, Fadista J, Spégel P, Metodiev MD, Larsson NG, Eliasson L, Wierup N, Mulder H (2014) Loss of TFB1M results in mitochondrial dysfunction that leads to impaired insulin secretion and diabetes. Hum Mol Genet 23(21):5733–5749. https://doi.org/10.1093/hmg/ddu288
Luo X, Li R, Yan LJ (2015) Roles of pyruvate, NADH, and mitochondrial complex I in redox balance and imbalance in β cell function and dysfunction. J Diabetes Res 2015:512618. https://doi.org/10.1155/2015/512618
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139(Suppl 1):216–231. https://doi.org/10.1111/jnc.13731
Park JS, Davis RL, Sue CM (2018) Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep 18(5):21. https://doi.org/10.1007/s11910-018-0829-3
Filichia E, Hoffer B, Qi X, Luo Y (2016) Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci Rep 6:32656. https://doi.org/10.1038/srep32656
Valdinocci D, Simões RF, Kovarova J, Cunha-Oliveira T, Neuzil J, Pountney DL (2019) Intracellular and intercellular mitochondrial dynamics in Parkinson’s disease. Front Neurosci 13:930. https://doi.org/10.3389/fnins.2019.00930
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106–107:17–32. https://doi.org/10.1016/j.pneurobio.2013.04.004
Schapira AH (2007) Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ 14(7):1261–1266. https://doi.org/10.1038/sj.cdd.4402160
Lev N, Roncevic D, Ickowicz D, Melamed E, Offen D (2006) Role of DJ-1 in Parkinson’s disease. J Mol Neurosci 29(3):215–225. https://doi.org/10.1385/jmn:29:3:215
Martin I, Kim JW, Dawson VL, Dawson TM (2014) LRRK2 pathobiology in Parkinson’s disease. J Neurochem 131(5):554–565. https://doi.org/10.1111/jnc.12949
Beal MF (2003) Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci 991:120–131. https://doi.org/10.1111/j.1749-6632.2003.tb07470.x
Kones R (2010) Parkinson’s disease: mitochondrial molecular pathology, inflammation, statins, and therapeutic neuroprotective nutrition. Nutr Clin Pract 25(4):371–389. https://doi.org/10.1177/0884533610373932
Bae JH, Jo SI, Kim SJ, Lee JM, Jeong JH, Kang JS, Cho NJ, Kim SS, Lee EY, Moon JS (2019) Circulating cell-free mtDNA contributes to AIM2 inflammasome-mediated chronic inflammation in patients with type 2 diabetes. Cells 8(4):328. https://doi.org/10.3390/cells8040328
Rasheed M, Liang J, Wang C, Deng Y, Chen Z (2021) Epigenetic regulation of neuroinflammation in Parkinson’s disease. Int J Mol Sci 22(9):4956. https://doi.org/10.3390/ijms22094956
Naoi M, Maruyama W, Shamoto-Nagai M (2020) Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinson’s disease. J Neural Transm (Vienna) 127(2):131–147. https://doi.org/10.1007/s00702-020-02150-w
Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100(5):1375–1386. https://doi.org/10.1111/j.1471-4159.2006.04327.x
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150(8):963–976. https://doi.org/10.1038/sj.bjp.0707167
Wu Y, Reece EA, Zhong J, Dong D, Shen WB, Harman CR, Yang P (2016) Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. Am J Obstet Gynecol 215(3):366.e1-366.e10. https://doi.org/10.1016/j.ajog.2016.03.036
Choi SK, Lim M, Yeon SI, Lee YH (2016) Inhibition of endoplasmic reticulum stress improves coronary artery function in type 2 diabetic mice. Exp Physiol 101(6):768–777. https://doi.org/10.1113/EP085508
Nomura J, Hosoi T, Kaneko M, Ozawa K, Nishi A, Nomura Y (2016) Neuroprotection by endoplasmic reticulum stress-induced HRD1 and chaperones: possible therapeutic targets for Alzheimer’s and Parkinson’s disease. Med Sci (Basel) 4(3):14. https://doi.org/10.3390/medsci4030014
Fonseca SG, Gromada J, Urano F (2011) Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab 22(7):266–274. https://doi.org/10.1016/j.tem.2011.02.008
Hu Y, Liu J, Yuan Y, Chen J, Cheng S, Wang H, Xu Y (2018) Sodium butyrate mitigates type 2 diabetes by inhibiting PERK-CHOP pathway of endoplasmic reticulum stress. Environ Toxicol Pharmacol 64:112–121. https://doi.org/10.1016/j.etap.2018.09.002
Natrus LV, Osadchuk YS, Lisakovska OO, Labudzinskyi DO, Klys YG, Chaikovsky YB (2022) Effect of propionic acid on diabetes-induced impairment of unfolded protein response signaling and astrocyte/microglia crosstalk in rat ventromedial nucleus of the hypothalamus. Neural Plast 22(2022):6404964. https://doi.org/10.1155/2022/6404964
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29(1):42–61. https://doi.org/10.1210/er.2007-0015
Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50(4):752–763. https://doi.org/10.1007/s00125-006-0590-z
Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M (2007) The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50(12):2486–2494. https://doi.org/10.1007/s00125-007-0816-8
Farombi EO, Awogbindin IO, Olorunkalu PD, Ogbuewu E, Oyetunde BF, Agedah AE, Adeniyi PA (2020) Kolaviron protects against nigrostriatal degeneration and gut oxidative damage in a stereotaxic rotenone model of Parkinson’s disease. Psychopharmacology 237(11):3225–3236. https://doi.org/10.1007/s00213-020-05605-w
Li XM, Zhang XJ, Dong MX (2017) Isorhynchophylline attenuates MPP+-induced apoptosis through endoplasmic reticulum stress- and mitochondria-dependent pathways in PC12 cells: involvement of antioxidant activity. Neuromolecular Med 19(4):480–492. https://doi.org/10.1007/s12017-017-8462
Ning B, Zhang Q, Wang N, Deng M, Fang Y (2019) β-Asarone regulates ER stress and autophagy via inhibition of the PERK/CHOP/Bcl-2/Beclin-1 pathway in 6-OHDA-induced parkinsonian rats. Neurochem Res 44(5):1159–1166. https://doi.org/10.1007/s11064-019-02757-w
Gorbatyuk MS, Shabashvili A, Chen W, Meyers C, Sullivan LF, Salganik M, Lin JH, Lewin AS, Muzyczka N, Gorbatyuk OS (2012) Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease. Mol Ther 20(7):1327–1337. https://doi.org/10.1038/mt.2012.28
Omura T, Kaneko M, Okuma Y, Orba Y, Nagashima K, Takahashi R, Fujitani N, Matsumura S, Hata A, Kubota K, Murahashi K, Uehara T, Nomura Y (2006) A ubiquitin ligase HRD1 promotes the degradation of Pael receptor, a substrate of Parkin. J Neurochem 99(6):1456–1469. https://doi.org/10.1111/j.1471-4159.2006.04155.x
Tong Y, Mukhamejanova Z, Zheng Y, Wen T, Xu F, Pang J (2021) Marine-derived xyloketal compound ameliorates MPP+-induced neuronal injury through regulating of the IRE1/XBP1 signaling pathway. ACS Chem Neurosci 12(16):3101–3111. https://doi.org/10.1021/acschemneuro.1c00362
Valdés P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, Martinez A, Galleguillos D, Armentano D, Schneider BL, Hetz C (2014) Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci U S A 111(18):6804–6809. https://doi.org/10.1073/pnas.1321845111
Sado M, Yamasaki Y, Iwanaga T, Onaka Y, Ibuki T, Nishihara S, Mizuguchi H, Momota H, Kishibuchi R, Hashimoto T, Wada D, Kitagawa H, Watanabe TK (2009) Protective effect against Parkinson’s disease-related insults through the activation of XBP1. Brain Res 1257:16–24. https://doi.org/10.1016/j.brainres.2008.11.104
Sun Y, Selvaraj S, Pandey S, Humphrey KM, Foster JD, Wu M, Watt JA, Singh BB, Ohm JE (2018) MPP+ decreases store-operated calcium entry and TRPC1 expression in mesenchymal stem cell derived dopaminergic neurons. Sci Rep 8(1):11715. https://doi.org/10.1038/s41598-018-29528-x
Wang T, Li X, Yang D, Zhang H, Zhao P, Fu J, Yao B, Zhou Z (2015) ER stress and ER stress-mediated apoptosis are involved in manganese-induced neurotoxicity in the rat striatum in vivo. Neurotoxicology 48:109–119. https://doi.org/10.1016/j.neuro.2015.02.007
Saha S, Panigrahi DP, Patil S, Bhutia SK (2018) Autophagy in health and disease: a comprehensive review. Biomed Pharmacother 104:485–495. https://doi.org/10.1016/j.biopha.2018.05.007
Rocha M, Apostolova N, Diaz-Rua R, Muntane J, Victor VM (2020) Mitochondria and T2D: role of autophagy, ER stress, and inflammasome. Trends Endocrinol Metab 31(10):725–741. https://doi.org/10.1016/j.tem.2020.03.004
Marasco MR, Linnemann AK (2018) β-Cell autophagy in diabetes pathogenesis. Endocrinology 159(5):2127–2141. https://doi.org/10.1210/en.2017-03273
Cheng Y, Ren X, Hait WN, Yang JM (2013) Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev 65(4):1162–1197. https://doi.org/10.1124/pr.112.007120
Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, Marselli L, Masiello P, Marchetti P (2009) Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 52(6):1083–1086. https://doi.org/10.1007/s00125-009-1347-2
Choi SK, Kwon Y, Byeon S, Lee YH (2020) Stimulation of autophagy improves vascular function in the mesenteric arteries of type 2 diabetic mice. Exp Physiol 105(1):192–200. https://doi.org/10.1113/EP087737
Quan W, Jung HS, Lee MS (2013) Role of autophagy in the progression from obesity to diabetes and in the control of energy balance. Arch Pharm Res 36(2):223–229. https://doi.org/10.1007/s12272-013-0024-7
Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, Zhou Z (2016) Vitamin D induces autophagy of pancreatic β-cells and enhances insulin secretion. Mol Med Rep 14(3):2644–2650. https://doi.org/10.3892/mmr.2016.5531
Olanow CW, Brundin P (2013) Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord 28(1):31–40. https://doi.org/10.1002/mds.25373
Stefanis L, Emmanouilidou E, Pantazopoulou M, Kirik D, Vekrellis K, Tofaris GK (2019) How is alpha-synuclein cleared from the cell? J Neurochem 150(5):577–590. https://doi.org/10.1111/jnc.14704
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305(5688):1292–1295. https://doi.org/10.1126/science
Bang Y, Kim KS, Seol W, Choi HJ (2016) LRRK2 interferes with aggresome formation for autophagic clearance. Mol Cell Neurosci 75:71–80. https://doi.org/10.1016/j.mcn.2016.06.007
Yue Z, Yang XW (2013) Dangerous duet: LRRK2 and α-synuclein jam at CMA. Nat Neurosci 16(4):375–377. https://doi.org/10.1038/nn.3361
Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147(4):893–906. https://doi.org/10.1016/j.cell.2011.10.018
Bozi LHM, Campos JC (2017) Targeting the ubiquitin proteasome system in diabetic cardiomyopathy. J Mol Cell Cardiol 109:61–63. https://doi.org/10.1016/j.yjmcc.2017.06.009
McKinnon C, De Snoo ML, Gondard E, Neudorfer C, Chau H, Ngana SG, O’Hara DM, Brotchie JM, Koprich JB, Lozano AM, Kalia LV, Kalia SK (2020) Early-onset impairment of the ubiquitin-proteasome system in dopaminergic neurons caused by α-synuclein. Acta Neuropathol Commun 8(1):17. https://doi.org/10.1186/s40478-020-0894-0
Kawaguchi M, Minami K, Nagashima K, Seino S (2006) Essential role of ubiquitin-proteasome system in normal regulation of insulin secretion. J Biol Chem 281(19):13015–13020. https://doi.org/10.1074/jbc.M601228200
Costes S, Vandewalle B, Tourrel-Cuzin C, Broca C, Linck N, Bertrand G, Kerr-Conte J, Portha B, Pattou F, Bockaert J, Dalle S (2009) Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in beta-cells and human pancreatic islets. Diabetes 58(5):1105–1115. https://doi.org/10.2337/db08-0926
Gao C, Huang W, Kanasaki K, Xu Y (2014) The role of ubiquitination and sumoylation in diabetic nephropathy. Biomed Res Int 2014:160692. https://doi.org/10.1155/2014/160692
Haataja L, Gurlo T, Huang CJ, Butler PC (2008) Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 29(3):303–316. https://doi.org/10.1210/er.2007-0037
Costes S (2018) Targeting protein misfolding to protect pancreatic beta-cells in type 2 diabetes. Curr Opin Pharmacol 43:104–110. https://doi.org/10.1016/j.coph.2018.08.016
Singh S, Trikha S, Sarkar A, Jeremic AM (2016) Proteasome regulates turnover of toxic human amylin in pancreatic cells. Biochem J 473(17):2655–2670. https://doi.org/10.1042/BCJ20160026
White MF (2002) IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283(3):E413–E422. https://doi.org/10.1152/ajpendo.00514.2001
Rui L, Yuan M, Frantz D, Shoelson S, White MF (2002) SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277(44):42394–42398. https://doi.org/10.1074/jbc.C200444200
Kim SJ, DeStefano MA, Oh WJ, Wu CC, Vega-Cotto NM, Finlan M, Liu D, Su B, Jacinto E (2012) mTOR complex 2 regulates proper turnover of insulin receptor substrate-1 via the ubiquitin ligase subunit Fbw8. Mol Cell 48(6):875–887. https://doi.org/10.1016/j.molcel.2012.09.029
Pelzer C, Cabalzar K, Wolf A, Gonzalez M, Lenz G, Thome M (2013) The protease activity of the paracaspase MALT1 is controlled by monoubiquitination. Nat Immunol 14(4):337–345. https://doi.org/10.1038/ni.2540
Joshi N, Raveendran A, Nagotu S (2020) Chaperones and proteostasis: role in Parkinson’s disease. Diseases 8(2):24. https://doi.org/10.3390/diseases8020024
Momtaz S, Memariani Z, El-Senduny FF, Sanadgol N, Golab F, Katebi M, Abdolghaffari AH, Farzaei MH, Abdollahi M (2020) Targeting ubiquitin-proteasome pathway by natural products: novel therapeutic strategy for treatment of neurodegenerative diseases. Front Physiol 28(11):361. https://doi.org/10.3389/fphys.2020.00361
Spratt DE, Martinez-Torres RJ, Noh YJ, Mercier P, Manczyk N, Barber KR, Aguirre JD, Burchell L, Purkiss A, Walden H, Shaw GS (2013) A molecular explanation for the recessive nature of parkin-linked Parkinson’s disease. Nat Commun 4:1983. https://doi.org/10.1038/ncomms2983
Giguère N, Pacelli C, Saumure C, Bourque MJ, Matheoud D, Levesque D, Slack RS, Park DS, Trudeau LÉ (2018) Comparative analysis of Parkinson’s disease-associated genes in mice reveals altered survival and bioenergetics of Parkin-deficient dopamine neurons. J Biol Chem 293(25):9580–9593. https://doi.org/10.1074/jbc.RA117.000499
Kawahata I, Fukunaga K (2020) Degradation of tyrosine hydroxylase by the ubiquitin-proteasome system in the pathogenesis of Parkinson’s disease and dopa-responsive dystonia. Int J Mol Sci 21(11):3779. https://doi.org/10.3390/ijms21113779.PMID:32471089
Yun JH, Lee DH, Jeong HS, Kim HS, Ye SK, Cho CH (2021) STAT3 activation in microglia exacerbates hippocampal neuronal apoptosis in diabetic brains. J Cell Physiol 236(10):7058–7070. https://doi.org/10.1002/jcp.30373
Cunha RA, Agostinho PM (2010) Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alzheimers Dis 20(Suppl 1):S95-116. https://doi.org/10.3233/JAD-2010-1408
Sánchez-Sarasúa S, Moustafa S, García-Avilés Á, López-Climent MF, Gómez-Cadenas A, Olucha-Bordonau FE, Sánchez-Pérez AM (2016) The effect of abscisic acid chronic treatment on neuroinflammatory markers and memory in a rat model of high-fat diet induced neuroinflammation. Nutr Metab (Lond) 26(13):73. https://doi.org/10.1186/s12986-016-0137-3
Infante-Garcia C, Jose Ramos-Rodriguez J, Marin-Zambrana Y, Teresa Fernandez-Ponce M, Casas L, Mantell C, Garcia-Alloza M (2017) Mango leaf extract improves central pathology and cognitive impairment in a type 2 diabetes mouse model. Brain Pathol 27(4):499–507. https://doi.org/10.1111/bpa.12433
Zhang PA, Sun Q, Li YC, Weng RX, Wu R, Zhang HH, Xu GY (2020) Overexpression of purinergic P2X4 receptors in hippocampus rescues memory impairment in rats with type 2 diabetes. Neurosci Bull 36(7):719–732. https://doi.org/10.1007/s12264-020-00478-7
Alzoubi KH, Mokhemer E, Abuirmeileh AN (2018) Beneficial effect of etazolate on depression-like behavior and learning, and memory impairment in a model of Parkinson’s disease. Behav Brain Res 17(350):109–115. https://doi.org/10.1016/j.bbr.2018.05.004
Kawashima S, Shimizu Y, Ueki Y, Matsukawa N (2021) Impairment of the visuospatial working memory in the patients with Parkinson’s disease: an fMRI study. BMC Neurol 21(1):335. https://doi.org/10.1186/s12883-021-02366-7
Souza MF, Medeiros KAAL, Lins LCRF, Bispo JMM, Gois AM, Freire MAM, Marchioro M, Santos JR (2020) Intracerebroventricular injection of deltamethrin increases locomotion activity and causes spatial working memory and dopaminergic pathway impairment in rats. Brain Res Bull 154:1–8. https://doi.org/10.1016/j.brainresbull.2019.10.002
Hanoğlu L, Ercan FB, Mantar N, Helvacı Yılmaz N, Sitrava S, Özer F, Yuluğ B (2019) Accelerated forgetting and verbal memory consolidation process in idiopathic nondement Parkinson’s disease. J Clin Neurosci 70:208–213. https://doi.org/10.1016/j.jocn.2019.08.012
Christopher L, Duff-Canning S, Koshimori Y, Segura B, Boileau I, Chen R, Lang AE, Houle S, Rusjan P, Strafella AP (2015) Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Ann Neurol 77(2):269–280. https://doi.org/10.1002/ana.24323
Guo Z, Ruan Z, Zhang D, Liu X, Hou L, Wang Q (2022) Rotenone impairs learning and memory in mice through microglia-mediated blood brain barrier disruption and neuronal apoptosis. Chemosphere 291(Pt 2):132982. https://doi.org/10.1016/j.chemosphere.2021.132982
Hou L, Sun F, Sun W, Zhang L, Wang Q (2019) Lesion of the locus coeruleus damages learning and memory performance in paraquat and maneb-induced mouse Parkinson’s disease model. Neuroscience 1(419):129–140. https://doi.org/10.1016/j.neuroscience.2019.09.006
Wang SY, Wu SL, Chen TC, Chuang CS (2020) Antidiabetic agents for treatment of Parkinson’s disease: a meta-analysis. Int J Environ Res Public Health 17(13):4805. https://doi.org/10.3390/ijerph17134805
Ahmad MH, Fatima M, Ali M, Rizvi MA, Mondal AC (2021) Naringenin alleviates paraquat-induced dopaminergic neuronal loss in SH-SY5Y cells and a rat model of Parkinson's disease. Neuropharmacology 201:108831. https://doi.org/10.1016/j.neuropharm.2021.108831
Mor DE, Sohrabi S, Kaletsky R, Keyes W, Tartici A, Kalia V, Miller GW, Murphy CT (2020) Metformin rescues Parkinson’s disease phenotypes caused by hyperactive mitochondria. Proc Natl Acad Sci U S A 117(42):26438–26447. https://doi.org/10.1073/pnas.2009838117
Ryu YK, Go J, Park HY, Choi YK, Seo YJ, Choi JH, Rhee M, Lee TG, Lee CH, Kim KS (2020) Metformin regulates astrocyte reactivity in Parkinson’s disease and normal aging. Neuropharmacology 15(175):108173. https://doi.org/10.1016/j.neuropharm.2020.108173
Paudel YN, Angelopoulou E, Piperi C, Shaikh MF, Othman I (2020) Emerging neuroprotective effect of metformin in Parkinson’s disease: a molecular crosstalk. Pharmacol Res 152:104593. https://doi.org/10.1016/j.phrs.2019.104593
Li Y, Li L, Hölscher C (2016) Incretin-based therapy for type 2 diabetes mellitus is promising for treating neurodegenerative diseases. Rev Neurosci 27(7):689–711. https://doi.org/10.1515/revneuro-2016-0018
Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T (2013) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 123(6):2730–2736. https://doi.org/10.1172/JCI68295
Hölscher C (2014) Drugs developed for treatment of diabetes show protective effects in Alzheimer’s and Parkinson’s diseases. Sheng Li Xue Bao 66(5):497–510
Sekar S, Taghibiglou C (2018) Elevated nuclear phosphatase and tensin homolog (PTEN) and altered insulin signaling in substantia nigral region of patients with Parkinson’s disease. Neurosci Lett 14(666):139–143. https://doi.org/10.1016/j.neulet.2017.12.049
Lopez Vicchi F, Luque GM, Brie B, Nogueira JP, Garcia Tornadu I, Becu-Villalobos D (2016) Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res 109:74–80. https://doi.org/10.1016/j.phrs.2015.12.029
Chang YH, Yen SJ, Chang YH, Wu WJ, Lin KD (2021) Pioglitazone and statins lower incidence of Parkinson disease in patients with diabetes mellitus. Eur J Neurol 28(2):430–437. https://doi.org/10.1111/ene.14542
Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB (2004) Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem 88(2):494–501. https://doi.org/10.1046/j.1471-4159.2003.02210.x
Struck LK, Rodnitzky RL, Dobson JK (1990) Stroke and its modification in Parkinson’s disease. Stroke 21(10):1395–1399. https://doi.org/10.1161/01.str.21.10.1395
Levine RL, Jones JC, Bee N (1992) Stroke and Parkinson’s disease. Stroke 23(6):839–42. https://doi.org/10.1161/01.str.23.6.839
Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, Mayeux R (2003) The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 14 60(1):87–93. https://doi.org/10.1212/wnl.60.1.87.24
Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD (2004) Parkinsonism in Ontario: comorbidity associated with hospitalization in a large cohort. Mov Disord 19(1):49–53. https://doi.org/10.1002/mds.10648
Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H (2006) Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism Relat Disor 12(3):185–189. https://doi.org/10.1016/j.parkreldis.2005.09.004
Leibson CL, Maraganore DM, Bower JH, Ransom JE, O’brien PC, Rocca WA, (2006) Comorbid conditions associated with Parkinson’s disease: a population-based study. Mov Disord 21(4):446–455. https://doi.org/10.1002/mds.20685
Scigliano G, Musicco M, Soliveri P, Piccolo I, Ronchetti G, Girotti F (2006) Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke 37(5):1184–1188. https://doi.org/10.1161/01.STR.0000217384.03237.9c
Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J (2007) Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 30(4):842–847. https://doi.org/10.2337/dc06-2011
Arvanitakis Z, Wilson RS, Bienias JL, Bennett DA (2007) Diabetes and parkinsonian signs in older persons. Alzheimer Dis Assoc Disord 21(2):144–149. https://doi.org/10.1097/WAD.0b013e31805ba768
Moran, L.B. and M.B.J.N. Graeber, Towards a pathway definition of Parkinson’s disease: a complex Moran LB, Graeber MB (2008). Towards a pathway definition of Parkinson’s disease: a complex disorder with links to cancer, diabetes and inflammation. Neurogenetics. 2008 Feb;9(1):1–13. https://doi.org/10.1007/s10048-007-0116-y.
Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G (2008) Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 31(10):2003–2005. https://doi.org/10.2337/dc08-0688
D’Amelio M, Ragonese P, Callari G, Di Benedetto N, Palmeri B, Terruso V, Salemi G, Famoso G, Aridon P, Savettieri G (2009) Diabetes preceding Parkinson’s disease onset. A case-control study Parkinsonism Relat Disord 15(9):660–664. https://doi.org/10.1016/j.parkreldis.2009.02.013
Menon R, Farina C (2011) Shared molecular and functional frameworks among five complex human disorders: a comparative study on interactomes linked to susceptibility genes. PLoS ONE 6(4):e18660. https://doi.org/10.1371/journal.pone.0018660
Palacios N, Gao X, McCullough ML, Jacobs EJ, Patel AV, Mayo T, Schwarzschild MA, Ascherio A (2011) Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord 26(12):2253–2259. https://doi.org/10.1002/mds.23855
Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B (2011) Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care. 34(5):1102–8. https://doi.org/10.2337/dc10-1333
Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY (2012) Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 35(5):1047–1049. https://doi.org/10.2337/dc11-1511
Wahlqvist ML, Lee MS, Hsu CC, Chuang SY, Lee JT, Tsai HN (2012) Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord 18(6):753–758. https://doi.org/10.1016/j.parkreldis.2012.03.010
Santiago JA, Potashkin JA (2013) Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS ONE 8(12):e83940. https://doi.org/10.1371/journal.pone.0083940
Santiago JA, Potashkin JA (2014). System-based approaches to decode the molecular links in Parkinson’s disease and diabetes. Neurobiol Dis 72 Pt A:84–91. https://doi.org/10.1016/j.nbd.2014.03.019.
Kotagal V, Albin RL, Müller ML, Koeppe RA, Frey KA, Bohnen NI (2013) Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat Disord 19(5):522–526. https://doi.org/10.1016/j.parkreldis.2013.01.016
De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner TT (2018) Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology 91(2):e139–e142. https://doi.org/10.1212/WNL.0000000000005771
Zhu Y, Pu J, Chen Y, Zhang B (2020) Decreased risk of Parkinson’s disease in diabetic patients with thiazolidinediones therapy: an exploratory meta-analysis. PLoS ONE 14(10):e0224236. https://doi.org/10.1371/journal.pone.0224236
Jeong SM, Han K, Kim D, Rhee SY, Jang W, Shin DW (2020) Body mass index, diabetes, and the risk of Parkinson’s disease. Mov Disord 35(2):236–244. https://doi.org/10.1002/mds.27922
Acknowledgements
The authors would like to acknowledge the School of Life Sciences, JNU.
Funding
ACM highly acknowledges the financial supports from DBT (BT/PR32907/MED/122/227/2019), Ministry of Science and Technology (Govt. of India). Institutional umbrella grants like DBT-BUILDER-Level-III (BT/INF/22/SP45382/2022), and DST-FIST-II to School of Life Sciences, JNU, New Delhi. Mr. Sandeep acknowledges the financial support from CSIR Grant (09/263(1219)/2019-EMR-I. MHA acknowledges the financial support from ICMR-SRF (45/07/2019/MP/BMS).
Author information
Authors and Affiliations
Contributions
ACM conceptualized, framed, and finally edited the manuscript; Sandeep, MHA, and LR wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Ethical approval is not required.
Consent for Publication
The authors have given consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
XXXX, S., Ahmad, M.H., Rani, L. et al. Convergent Molecular Pathways in Type 2 Diabetes Mellitus and Parkinson’s Disease: Insights into Mechanisms and Pathological Consequences. Mol Neurobiol 59, 4466–4487 (2022). https://doi.org/10.1007/s12035-022-02867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02867-7