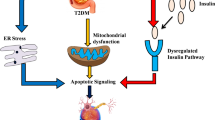
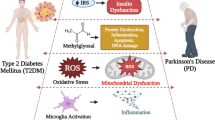
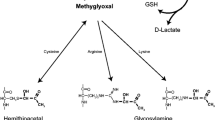
This review discusses the epidemiological, clinical, and pathophysiological aspects of the relationship between Parkinson’s disease (PD) and type 2 diabetes mellitus (DM2). DM2 increases the risk of developing PD and correlates with the rate of progression of PD and the severity of motor and cognitive deficits. At the same time, insulin resistance may operate as a key link in the pathogenesis of both diseases: peripherally in DM2 and in brain tissues in PD. In this regard, the use of antidiabetic drugs as neuroprotective agents opens up opportunities for developing a new targeted therapy strategy which modifies the course of PD.
Similar content being viewed by others
References
Mhyre, T. R., Boyd, J. T., Hamill, R. W., and Maguire-Zeiss, K. A., “Parkinson’s disease,” Subcell. Biochem., 65, 389–455 (2012), https://doi.org/10.1007/978-94-007-5416-4_16.
GBD 2016 Parkinson’s Disease Collaborators, “Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016,” Lancet Neurol., 17, No. 11, 939–953 (2018), https://doi.org/10.1016/S1474-4422(18)30295-3 (published correction appears in Lancet Neurol., 20, No. 12, e7 (2021)).
Illarioshkin, S. N., “Current views on the etiology of Parkinson’s disease,” Nevrol. Zh., 20, No. 4, 4–13 (2015), https://doi.org/10.18821/1560-9545-2015-20-4-4-13.
Veryugina, N. I., Levin, O. S., and Lyashenko, E. A., “Neuroendocrine and metabolic impairments in Parkinson’s disease,” Zh. Nevrol. Psikhiatr., 120, Spec. Iss., No. 10–2, 67–73 (2020), https://doi.org/10.17116/jnevro202012010267.
Hassan, A., Sharma Kandel, R., et al., “Diabetes mellitus and Parkinson’s disease: Shared pathophysiological links and possible therapeutic implications,” Cureus, 12, No. 8, e9853 (2020), https://doi.org/10.7759/cureus.9853.
Athauda, D. and Foltynie, T., “Insulin resistance and Parkinson’s disease: A new target for disease modification?” Prog. Neurobiol., 145– 146, 98–120 (2016), https://doi.org/10.1016/j.pneurobio.2016.10.001.
Aviles-Olmos, I., Limousin, P., Lees, A., and Foltynie, T., “Parkinson’s disease, insulin resistance and novel agents of neuroprotection,” Brain, 136, No. 2, 374–384 (2013), https://doi.org/10.1093/brain/aws009.
Santiago, J. A. and Potashkin, J. A., “Shared dysregulated pathways lead to Parkinson’s disease and diabetes,” Trends Mol. Med., 9, 176– 186 (2013), https://doi.org/10.1016/j.molmed.2013.01.002.
Craft, S. and Watson, G. S., “Insulin and neurodegenerative disease: Shared and specific mechanisms,” Lancet Neurol., 3, 169–178 (2004), https://doi.org/10.1016/s1474-4422(04)00681-7.
Biosa, A., Outeiro, T. F., Bubacco, L., and Bisaglia, M., “Diabetes mellitus as a risk factor for Parkinson’s disease: A molecular point of view,” Mol. Neurobiol., 55, 8754–8763 (2018), https://doi.org/10.1007/s12035-018-1025-9.
Hölscher, C., “Brain insulin resistance: role in neurodegenerative disease and potential for targeting,” Expert Opin. Investig. Drugs, 29, No. 4, 333–348 (2020), https://doi.org/10.1080/13543784.2020.1738383.
Kabir, M., Ferdous Mitu, J., Akter, R., et al., “Therapeutic potential of dopamine agonists in the treatment of type 2 diabetes mellitus,” Environ. Sci. Pollut. Res. Int., 29, No. 31, 46385–46404 (2022), https://doi.org/10.1007/s11356-022-20445-1.
Pagano, G., Polychronis, S., Wilson, H., et al., “Diabetes mellitus and Parkinson disease,” Neurology, 90, No. 19, e1654–e1662 (2018), https://doi.org/10.1212/wnl.0000000000005475.
Ben-Joseph, A., Haque, T., Gallagher, D., et al., “Type 2 diabetes mellitus may worsen severity of motor symptoms in people with Parkinson’s disease [abstract],” Mov. Disord., 35, Suppl. 1, S153 (2020), https://doi.org/10.1002/mds.28268.
Hussein, M., Khamis, A., Soliman, R., and Ali, S., “Metabolic syndrome and insulin resistance in Parkinson’s disease: could they affect motor or cognitive symptoms? [abstract],” Mov. Disord., 34, Suppl. 2, (2019), https://www.mdsabstracts.org/abstract/metabol-ic-syndrome-and-insulin-resistance-in-parkinsons-disease-could-they-affect-motor-or-cognitive-symptoms, https://doi.org/10.1002/mds.v34.s2, acc. Sept. 18, 2021.
Palacios, N., Gao, X., McCullough, M. L., et al., “Obesity, diabetes, and risk of Parkinson’s disease,” Mov. Disord., 26, No. 12, 2253– 2259 (2011), https://doi.org/10.1002/mds.23855.
Lu, L., Fu, D., Li, H., et al., “Diabetes and risk of Parkinson’s disease: An updated meta-analysis of case-control studies,” PLoS One, 9, No. 1, e85781 (2014), https://doi.org/10.1371/journal.pone.0085781.
Cheong, J., de Pablo-Fernandez, E., Foltynie, T., and Noyce, A., “The association between type 2 diabetes mellitus and Parkinson’s disease,” J. Parkinsons Dis., 10, No. 3, 775–789 (2020), https://doi.org/10.3233/jpd-191900.
De Iuliis, A., Montinaro, E., Fatati, G., et al., “Diabetes mellitus and Parkinson’s disease: dangerous liaisons between insulin and dopamine,” Neural Regen. Res., 17, No. 3, 523–533 (2022), https://doi.org/10.4103/1673-5374.320965.
Martinez-Valbuena, I., Valenti-Azcarate, R., Amat-Villegas, I., et al., “Mixed pathologies in pancreatic β cells from subjects with neurodegenerative diseases and their interaction with prion protein,” Acta Neuropathol. Commun., 9, No. 1, Article 64 (2021), https://doi.org/10.1186/s40478-021-01171-0.
Delamarre, A., Rigalleau, V., and Meissner, W., “Insulin resistance, diabetes and Parkinson’s disease: The match continues,” Parkinsonism Relat. Disord., 80, 199–200 (2020), https://doi.org/10.1016/j.parkreldis.2020.10.013.
Mollenhauer, B., Zimmermann, J., Sixel-Döring, F., et al., “Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa),” Mov. Disord., 34, No. 1, 67–77 (2018), https://doi.org/10.1002/mds.27492.
Mucibabic, M., Steneberg, P., Lidh, E., et al., “α-Synuclein promotes IAPP fibril formation in vitro and β-cell amyloid formation in vivo in mice,” Sci. Rep., 10, No. 1, Art. 20438 (2020), https://doi.org/10.1038/s41598-020-77409-z.
Elabi, O. F., Cunha, J. P. M. C. M., Gaceb, A., et al., “High-fat diet-induced diabetes leads to vascular alterations, pericyte reduction, and perivascular depletion of microglia in a 6-OHDA toxin model of Parkinson disease,” J. Neuroinflammation, 18, No. 1, 175 (2021), https://doi.org/10.1186/s12974-021-02218-8.
Larsen, M. E. C., Thykjaer, A. S., Pedersen, F. N., et al., “Diabetic retinopathy as a potential marker of Parkinson’s disease: A register-based cohort study,” Brain Commun., 3, No. 4, fcab262 (2021), https://doi.org/10.1093/braincomms/fcab262.
Mauricio, D., Vlacho, B., Barrot de la Puente, J., et al., “Associations between diabetic retinopathy and Parkinson’s disease: Results from the Catalonian Primary Care Cohort Study,” Front. Med (Lausanne), 8, 800973 (2022), https://doi.org/10.3389/fmed.2021.800973.
Lv Y-Q, Yuan, L., Sun, Y., et al., “Long-term hyperglycemia aggravates α-synuclein aggregation and dopaminergic neuronal loss in a Parkinson’s disease mouse model,” Transl. Neurodegener., 11, No. 1, 14 (2022), https://doi.org/10.1186/s40035-022-00288-z.
De Pablo-Fernández, E., Courtney, R., Rockliffe, A., et al., “Faster disease progression in Parkinson’s disease with type 2 diabetes is not associated with increased α-synuclein, tau, amyloid-β or vascular pathology,” Neuropathol. Appl. Neurobiol., 47, No. 7, 1080–1091 (2021), https://doi.org/10.1111/nan.12728.
Milstein, J. and Ferris, H., “The brain as an insulin-sensitive metabolic organ,” Mol. Metab., 52, 101234 (2021), https://doi.org/10.1016/j.molmet.2021.101234.
Fiory, F., Perruolo, G., Cimmino, I., et al., “The relevance of insulin action in the dopaminergic system,” Front. Neurosci., 13, Art. 868 (2019), https://doi.org/10.3389/fnins.2019.00868.
Spinelli, M., Fusco, S., and Grassi, C., “Brain insulin resistance and hippocampal plasticity: Mechanisms and biomarkers of cognitive decline,” Front. Neurosci., 13, 788 (2019), https://doi.org/10.3389/fnins.2019.00788.
Duarte, A., Moreira, P., and Oliveira, C., “Insulin in central nervous system: More than just a peripheral hormone,” J. Aging Res., 2012, 1–21 (2012), https://doi.org/10.1155/2012/384017.
Pomytkin, I. and Pinelis, V., “Brain insulin resistance: focus on insulin receptor-mitochondria interactions,” Life (Basel), 11, No. 3, 262 (2021), https://doi.org/10.3390/life11030262.
Ametov, A. S., Type 2 Diabetes Mellitus. Problems and Solutions, GEOTAR-Media, Moscow (2014), ISBN: 978-5-9704-2829-0, https://www.rosmedlib.ru/book/ISBN9785970428290.html, acc. Sept. 30, 2021.
Woert, M. and Mueller, P., “Glucose, insulin, and free fatty acid metabolism in Parkinson’s disease treated with levodopa,” Clin. Pharmacol. Ther., 12, No. 2, Part 2, 360–367 (1971), https://doi.org/10.1002/cpt1971122part2360.
Sandyk, R., “The relationship between diabetes mellitus and Parkinson’s disease,” Int. J. Neurosci., 69, No. 1–4, 125–130 (1993), https://doi.org/10.3109/00207459309003322.
Marques, A., Dutheil, F., Durand, E., et al., “Glucose dysregulation in Parkinson’s disease: Too much glucose or not enough insulin?” Parkinsonism Relat. Disord., 55, 122–127 (2018), https://doi.org/10.1016/j.parkreldis.2018.05.026.
Vikdahl, M., Carlsson, M., Linder, J., et al., “Weight gain and increased central obesity in the early phase of Parkinson’s disease,” Clin. Nutr., 33, No. 6, 1132–1139 (2014), https://doi.org/10.1016/j.clnu.2013.12.012.
Morales-Briceño, H., Cervantes-Arriaga, A., Rodríguez-Violante, M., et al., “Overweight is more prevalent in patients with Parkinson’s disease,” Arq. Neuropsiquiatr., 70, No. 11, 843–846 (2012), https://doi.org/10.1590/s0004-282x2012001100004.
Petroni, M., Albani, G., Bicchiega, V., et al., “Body composition in advanced-stage Parkinson’s disease,” Acta Diabetol., 40, No. 0, s187-s190 (2003), https://doi.org/10.1007/s00592-003-0062-6.
Femat-Roldán, G., Gaitán Palau, M., Castilla-Cortázar, I., et al., “Altered body composition and increased resting metabolic rate associated with the postural instability/gait difficulty Parkinson’s disease subtype,” Parkinsons Dis., 2020, 1–9 (2020), https://doi.org/10.1155/2020/8060259.
Barichella, M., Marczewska, A., Mariani, C., et al., “Body weight gain rate in patients with Parkinson’s disease and deep brain stimulation,” Mov. Disord., 18, No. 11, 1337–1340 (2003), https://doi.org/10.1002/mds.10543.
Hogg, E., Athreya, K., Basile, C., et al., “High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson’s disease,” J. Parkinsons Dis., 8, No. 2, 259–265 (2018), https://doi.org/10.3233/jpd-181305.
Clegg, D. J., Gotoh, K., Kemp, C., et al., “Consumption of a high-fat diet induces central insulin resistance independent of adiposity,” Physiol. Behav., 103, 10–16 (2011), https://doi.org/10.1016/j.physbeh.2011.01.010.
Prasuhn, J., Davis, R., and Kumar, K., “Targeting mitochondrial impairment in Parkinson’s disease: Challenges and opportunities,” Front. Cell. Dev. Biol., 8, (2021), https://doi.org/10.3389/fcell.2020.615461.
Salmina, A. B., Yauzina, N. A., Kuvacheva, N. V., et al., “Insulin and insulin resistance: new molecular markers and target molecules for the diagnosis and therapy of diseases of the central nervous system,” Byull. Sibirsk. Med., 12, No. 5, 104–118 (2013), https://doi.org/10.20538/1682-0363-2013-5-104-118.
Rhea, E. M. and Banks, W. A., “Role of the blood–brain barrier in central nervous system insulin resistance,” Front. Neurosci., 13, 521 (2019), https://doi.org/10.3389/fnins.2019.00521.
Wu, Y. C., Sonninen, T. M., Peltonen, S., et al., “Blood–brain barrier and neurodegenerative diseases – modeling with iPSC-derived brain cells,” Int. J. Mol. Sci., 22, No. 14, 7710 (2021), https://doi.org/10.3390/ijms22147710.
Hou, X., Watzlawik, J., Fiesel, F., and Springer, W., “Autophagy in Parkinson’s disease,” J. Mol. Biol., 432, No. 8, 2651–2672 (2020), https://doi.org/10.1016/j.jmb.2020.01.037.
Liu, X. L., Wang, Y. D., Yu, X. M., et al., “Mitochondria-mediated damage to dopaminergic neurons in Parkinson’s disease (Review),” Int. J. Mol. Med., 41, No. 2, 615–623 (2018), https://doi.org/10.3892/ijmm.2017.3255.
Sripetchwandee, J., Chattipakorn, N., and Chattipakorn, S. C., “Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia,” Front. Endocrinol (Lausanne), 9, 496 (2018), https://doi.org/10.3389/fendo.2018.00496.
Tagliati, M., Hogg, E., Wu, T., et al., “Central insulin resistance index is independent of peripheral insulin resistance in Parkinson’s disease [abstract],” Mov. Disord., 34, Suppl. 2 (2019), https://www.mdsabstracts.org/abstract/central-insulin-resistance-index-is-inde-pendent-of-peripheral-insulin-resistance-in-parkinsons-disease, https://doi.org/10.1002/mds.v34.s2, acc. Sept. 6, 2021.
Kleinridders, A., Cai, W., Cappellucci, L., et al., “Insulin resistance in brain alters dopamine turnover and causes behavioral disorders,” Proc. Natl. Acad. Sci. USA, 112, No. 11, 3463–3468 (2015), https://doi.org/10.1073/pnas.1500877112.
Gao, S., Duan, C., Gao, G., et al., “Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling,” Int. J. Biochem. Cell. Biol., 64, 25–33 (2015), https://doi.org/10.1016/j.biocel.2015.03.006.
Iravanpour, F., Dargahi, L., Rezaei, M., et al., “Intranasal insulin improves mitochondrial function and attenuates motor deficits in a rat 6-OHDA model of Parkinson’s disease,” CNS Neurosci. Ther., 27, No. 3, 308–319 (2021), https://doi.org/10.1111/cns.13609.
Sharma, S. K., Chorell, E., Steneberg, P., et al., “Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner,” Sci. Rep., 5, 12531 (2015), https://doi.org/10.1038/srep12531.
Cereda, E., Barichella, M., Cassani, E., et al., “Clinical features of Parkinson disease when onset of diabetes came first: A case-control study,” Neurology, 78, No. 19, 1507–1511 (2012), https://doi.org/10.1212/wnl.0b013e3182553cc9.
Chan, H., Cheung, Y., Chau, D., et al., “Metabolic syndrome: its link with motor function of Parkinson’s disease [abstract],” Mov. Disord., 32, Suppl. 2, Abstr. 522 (2017), https://doi.org/10.1002/mds.27087.
Zittel, S., Uyar, M., Lezius, S., et al., “HbA1c and motor outcome in Parkinson’s disease in the Mark-PD Study,” Mov. Disord., 36, No. 8, 1991–1992 (2021), https://doi.org/10.1002/mds.28689.
Kotagal, V., Albin, R., Müller, M., et al., “Diabetes is associated with postural instability and gait difficulty in Parkinson disease,” Parkinsonism Relat. Disord., 19, No. 5, 522–526 (2013), https://doi.org/10.1016/j.parkreldis.2013.01.016.
Sharma, T., Kaur, D., Grewal, A. K., and Singh, T. G., “Therapies modulating insulin resistance in Parkinson’s disease: A cross talk,” Neurosci. Lett., 749, 135754 (2021), https://doi.org/10.1016/j.neulet.2021.135754.
Meléndez-Flores, J., Castillo-Torres, S., Cerda-Contreras, C., et al., “Clinical features of metabolic syndrome in patients with Parkinson’s disease,” Revista de Neurología, 72, No. 01, 9–15 (2021), https://doi.org/10.33588/rn.7201.2020323.
Chohan, H., Senkevich, K., Patel, R., et al., “Type 2 diabetes as a determinant of Parkinson’s disease risk and progression,” Mov. Disord., 36, No. 6, 1420–1429 (2021), https://doi.org/10.1002/mds.28551.
Ashraghi, M. R., Pagano, G., Polychronis, S., et al., “Parkinson’s disease, diabetes and cognitive impairment,” Recent Pat Endocr. Metab. Immune Drug Discov., 10, 11–21 (2016), https://doi.org/10.2174/1872214810999160628105549.
Bergantin, L. B., “A link between brain insulin resistance and cognitive dysfunctions: Targeting Ca2+/cAMP signalling,” Cent. Nerv. Syst. Agents Med. Chem., 20, No. 2, 103–109 (2020), https://doi.org/10.2174/1871524920666200129121232.
Willmann, C., Brockmann, K., Wagner, R., et al., “Insulin sensitivity predicts cognitive decline in individuals with prediabetes,” BMJ Open Diabetes Res. Care, 8, No. 2, e001741 (2020), https://doi.org/10.1136/bmjdrc-2020-001741.
Yoo, H., Chung, S., Lee, P., et al., “The influence of body mass index at diagnosis on cognitive decline in Parkinson’s disease,” J. Clin. Neurol., 15, No. 4, 517 (2019), https://doi.org/10.3988/jcn.2019.15.4.517.
Kim, H., Oh, E., Lee, J., et al., “Relationship between changes of body mass index (BMI) and cognitive decline in Parkinson’s disease (PD),” Arch. Gerontol. Geriatr., 55, No. 1, 70–72 (2012), https://doi.org/10.1016/j.archger.2011.06.022.
Lorefalt, B., Ganowiak, W., Palhagen, S., et al., “Factors of importance for weight loss in elderly patients with Parkinson’s disease,” Acta Neurol. Scand., 110, No. 3, 180–187 (2004), https://doi.org/10.1111/j.1600-0404.2004.00307.x.
Wills, A., Li, R., Pérez, A., et al., “Predictors of weight loss in early treated Parkinson’s disease from the NET-PDLS-1 cohort,” J. Neurol., 264, No. 8, 1746–1753 (2017), https://doi.org/10.1007/s00415-017-8562-4.
Yang, L., Wang, H., Liu, L., and Xie, A., “The role of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson’s disease dementia,” Front. Neurosci., 12, Art. 73 (2018), https://doi.org/10.3389/fnins.2018.00073.
Bosco, D., Plastino, M., Cristiano, D., et al., “Dementia is associated with insulin resistance in patients with Parkinson’s disease,” J. Neurol. Sci., 315, No. 1–2, 39–43 (2012), https://doi.org/10.1016/j.jns.2011.12.008.
Gan’kina, O. A., Levin, O. S., and Il’yasova, F. N., “Cognitive impairment in patients with Metabolic syndrome,” Effekt. Farmakoter. Endokrinol., 29, No. 3, 16–20 (2016), https://umedp.ru/upload/iblock/2a8/endo_03_2016.pdf, acc. Sept. 30, 2021.
Bergmans, R., Rapp, A., Kelly, K., et al., “Understanding the relationship between type 2 diabetes and depression: lessons from genetically informative study designs,” Diabetes Med., 38, No. 2, e14399 (2020), https://doi.org/10.1111/dme.14399.
Riederer, P., Bartl, J., Laux, G., and Grünblatt, E., “Diabetes type II: A risk factor for depression–Parkinson–Alzheimer?” Neurotox. Res., 19, No. 2, 253–265 (2010), https://doi.org/10.1007/s12640-010-9203-1.
Roh, J., Lee, S., and Yoon, J., “Metabolic syndrome and Parkinson’s disease incidence: A nationwide study using propensity score matching,” Metab. Syndr. Relat. Disord., 19, No. 1, 1–7 (2021), https://doi.org/10.1089/met.2020.0060.
Nam, G., Kim, S., Han, K., et al., “Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study,” PLoS Med., 15, No. 8, e1002640 (2018), https://doi.org/10.1371/journal.pmed.1002640.
Sääksjärvi, K., Knekt, P., Männistö, S., et al., “Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease,” Parkinsonism Relat. Disord., 21, No. 10, 1148–1155 (2015), https://doi.org/10.1016/j.parkreldis.2015.07.017.
Maraki, M. I., Yannakoulia, M., Stamelou, M., et al., “Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease,” Mov. Disord., 34, No. 1, 48–57 (2019), https://doi.org/10.1002/mds.27489.
Cassani, E., Barichella, M., Ferri, V., et al., “Dietary habits in Parkinson’s disease: Adherence to Mediterranean diet,” Parkinsonism Relat. Disord., 42, 40–46 (2017), https://doi.org/10.1016/j.parkreldis.2017.06.007.
Fisher, B. E., Wu, A. D., Salem, G. J., et al., “The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease,” Arch. Phys. Med. Rehabil., 89, No. 7, 1221–1229 (2008), https://doi.org/10.1016/j.apmr.2008.01.013.
Hölscher, C., “First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer’s disease,” Alzheimers Dement., 10, No. 1 Suppl., S33–S37 (2014), https://doi.org/10.1016/j.jalz.2013.12.006.
Hölscher, C., “Insulin signaling impairment in the brain as a risk factor in Alzheimer’s disease,” Front. Aging Neurosci., 11, Art. 88 (2019), https://doi.org/10.3389/fnagi.2019.00088.
Yu, Y. W., Hsueh, S. C., Lai, J. H., et al., “Glucose-dependent insulinotropic polypeptide mitigates 6-OHDA-induced behavioral impairments in Parkinsonian rats,” Int. J. Mol. Sci., 19, No. 4, 1153 (2018), https://doi.org/10.3390/ijms19041153.
Vaccari, C., Grotto, D., Pereira, T., et al., “GLP-1 and GIP receptor agonists in the treatment of Parkinson’s disease: Translational systematic review and meta-analysis protocol of clinical and preclinical studies,” PLoS One, 16, No. 8, e0255726 (2021), https://doi.org/10.1371/journal.pone.0255726.
Zhou, M., Chen, S., Peng, P., et al., “Dulaglutide ameliorates STZ induced AD-like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3β,” Biochem. Biophys. Res. Commun., 511, No. 1, 154–160 (2019), https://doi.org/10.1016/j.bbrc.2019.01.103.
Wadden, T. A., Hollander, P., Klein, S., et al., “Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study,” Int. J. Obes (Lond.), 37, No. 11, 1443–1451 (2013), 10. 1038/ijo.2013.120 (published correction appears in Int. J. Obes. (Lond.), 39, No. 1, 187 (2015)).
Hamilton, A. and Hölscher, C., “Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system,” Neuroreport, 20, No. 13, 1161–1166 (2009), https://doi.org/10.1097/WNR.0b013e32832fbf14.
Baggio, L. L. and Drucker, D. J., “Biology of incretins: GLP-1 and GIP,” Gastroenterology, 132, No. 6, 2131–2157 (2007), https://doi.org/10.1053/j.gastro.2007.03.054.
Zhang, L., Li, L., and Hölscher, C., “Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of Parkinson’s disease,” J. Parkinsons Dis., 9, No. 1, 157–171 (2019), https://doi.org/10.3233/jpd-181503.
Grieco, M., Giorgi, A., Gentile, M., et al., “Glucagon-like peptide-1: A focus on neurodegenerative diseases,” Front. Neurosci., 13, Art. 1112 (2019), https://doi.org/10.3389/fnins.2019.01112.
Liu, W., Jalewa, J., Sharma, M., et al., “Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease,” Neuroscience, 303, 42–50 (2015), https://doi.org/10.1016/j.neuroscience.2015.06.054.
Aviles-Olmos, I., Dickson, J., Kefalopoulou, Z., et al., “Exenatide and the treatment of patients with Parkinson’s disease,” J. Clin. Invest., 123, No. 6, 2730–2736 (2013), https://doi.org/10.1172/JCI68295.
Aviles-Olmos, I., Dickson, J., Kefalopoulou, Z., et al., “Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease,” J. Parkinsons Dis., 4, No. 3, 337–344 (2014), https://doi.org/10.3233/JPD-140364.
Athauda, D., Maclagan, K., Skene, S., et al., “Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial,” Lancet, 390, No. 10103, 1664–1675 (2017), https://doi.org/10.1016/s0140-6736(17)31585-4.
Zhu, Y., Pu, J., Chen, Y., and Zhang, B., “Decreased risk of Parkinson’s disease in diabetic patients with thiazolidinediones therapy: An exploratory meta-analysis,” PLoS One, 14, No. 10, e0224236 (2019), https://doi.org/10.1371/journal.pone.0224236.
Brakedal, B., Flønes, I., Reiter, S. F., et al., “Glitazone use associated with reduced risk of Parkinson’s disease,” Mov. Disord., 32, No. 11, 1594–1599 (2017), https://doi.org/10.1002/mds.27128.
Wu, H. F., Kao, L. T., Shih, J. H., et al., “Pioglitazone use and Parkinson’s disease: A retrospective cohort study in Taiwan,” BMJ Open,8,No.8,e023302(2018),https://doi.org/10.1136/bmjopen-2018-023302.
Saunders, A., Burns, D., and Gottschalk, W., “Reassessment of pioglitazone for Alzheimer’s disease,” Front. Neurosci., 15, Art. 666958 (2021), https://doi.org/10.3389/fnins.2021.666958.
NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators, “Pioglitazone in early Parkinson’s disease: A phase 2, multicentre, double-blind, randomised trial,” Lancet Neurol., 14, No. 8, 795–803 (2015), https://doi.org/10.1016/S1474-4422(15)00144-1 (published correction appears in Lancet Neurol., 14, No. 8, 979 (2015)).
Valencia, W., Palacio, A., Tamariz, L., and Florez, H., “Metformin and ageing: improving ageing outcomes beyond glycaemic control,” Diabetologia, 60, No. 9, 1630–1638 (2017), https://doi.org/10.1007/s00125-017-4349-5.
Łabuzek, K., Suchy, D., Gabryel, B., et al., “Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide,” Pharmacol. Rep., 62, No. 5, 956–965 (2010), https://doi.org/10.1016/s1734-1140(10)70357-1.
Shi, Q., Liu, S., Fonseca, V., et al., “Effect of metformin on neuro-degenerative disease among elderly adult US veterans with type 2 diabetes mellitus,” BMJ Open, 9, No. 7, e024954 (2019), https://doi.org/10.1136/bmjopen-2018-024954.
Sportelli, C., Urso, D., Jenner, P., and Chaudhuri, K., “Metformin as a potential neuroprotective agent in prodromal Parkinson’s disease – viewpoint,” Front. Neurol., 11, Art. 556 (2020), https://doi.org/10.3389/fneur.2020.00556.
Aroda, V., Edelstein, S., Goldberg, R., et al., “Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study,” J. Clin. Endocrinol. Metab., 101, No. 4, 1754– 1761 (2016), https://doi.org/10.1210/jc.2015-3754.
Christine, C., Auinger, P., Saleh, N., et al., “Relationship of cerebrospinal fluid vitamin B12 status markers with Parkinson’s disease progression,” Mov. Disord., 35, No. 8, 1466–1471 (2020), https://doi.org/10.1002/mds.28073.
Hunter, K. and Hölscher, C., “Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis,” BMC Neurosci., 13, No. 1, Art. 33 (2012), https://doi.org/10.1186/1471-2202-13-33.
Yuan, Z., Li, D., Feng, P., et al., “A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease,” Eur. J. Pharmacol., 812, 82–90 (2017), https://doi.org/10.1016/j.ejphar.2017.06.029.
Finan, B., Ma, T., Ottaway, N., et al., “Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans,” Sci. Transl. Med., 5, No. 209, 209ra151-209ra151 (2013), https://doi.org/10.1126/scitranslmed.3007218.
Ji, C., Xue, G., Lijun, C., et al., “A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson's disease by increasing expression of BDN,” Brain Res., 1634, 1–11 (2016), https://doi.org/10.1016/j.brainres.2015.09.035.
Shi, L., Zhang, Z., Li, L., and Hölscher, C., “A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer icv. STZ rat model,” Behav. Brain Res., 327, 65–74 (2017), https://doi.org/10.1016/j.bbr.2017.03.032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 122, No. 11, Iss. 2, pp. 12–18, November, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Troshneva, A.Y., Ametov, A.S. Parkinson’s Disease and Type 2 Diabetes Mellitus: Interrelated Pathogenetic Mechanisms and Common Therapeutic Approaches. Neurosci Behav Physi 53, 959–965 (2023). https://doi.org/10.1007/s11055-023-01488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01488-4