Abstract
Astrocytes may undergo a functional remodeling with aging, acquiring a pro-inflammatory state. In line with this, resveratrol represents an interesting strategy for a healthier brain aging since it can improve glial functions. In the present study, we investigated the glioprotective role of resveratrol against lipopolysaccharide (LPS)-induced gliotoxicity in hippocampal aged astrocytes. Astrocyte cultures were obtained from aged rats (365 days old) and challenged in vitro with LPS in the presence of resveratrol. Cultured astrocytes from newborn rats were used as an age comparative for evaluating LPS gliotoxicity. In addition, aged rats were submitted to an acute systemic inflammation with LPS. Hippocampal astrocyte cultures were also obtained from these LPS-stimulated aged animals to further investigate the glioprotective effects of resveratrol in vitro. Overall, our results show that LPS induced a higher inflammatory response in aged astrocytes, compared to newborn astrocytes. Several inflammatory and gene expression alterations promoted by LPS in aged astrocyte cultures were similar in hippocampal tissue from aged animals submitted to in vivo LPS injection, corroborating our in vitro findings. Resveratrol, in turn, presented anti-inflammatory effects in aged astrocyte cultures, which were associated with downregulation of p21 and pro-inflammatory cytokines, Toll-like receptors (TLRs), and nuclear factor κB (NFκB). Resveratrol also improved astroglial functions. Upregulation of sirtuin 1 (SIRT1), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase 1 (HO-1) represent potential molecular mechanisms associated with resveratrol-mediated glioprotection. In summary, our data show that resveratrol can prime aged astrocytes against gliotoxic stimuli, contributing to a healthier brain aging.









Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Partridge L, Deelen J, Slagboom PE (2018) Facing up to the global challenges of ageing. Nature 561:45–56. https://doi.org/10.1038/s41586-018-0457-8
López-Otín C, Blasco MA, Partridge L et al (2013) The Hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Shivarama Shetty M, Sajikumar S (2017) ‘Tagging’ along memories in aging: synaptic tagging and capture mechanisms in the aged hippocampus. Ageing Res Rev 35:22–35. https://doi.org/10.1016/j.arr.2016.12.008
Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7:30–40. https://doi.org/10.1038/nrn1809
Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69:143–179. https://doi.org/10.1016/S0301-0082(02)00126-0
Valori CF, Guidotti G, Brambilla L, Rossi D (2019) Astrocytes: emerging therapeutic targets in neurological disorders. Trends Mol Med 25:750–759. https://doi.org/10.1016/j.molmed.2019.04.010
Mederos S, González-Arias C, Perea G (2018) Astrocyte–neuron networks: a multilane highway of signaling for homeostatic brain function. Front Synaptic Neurosci 10:45. https://doi.org/10.3389/fnsyn.2018.00045
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. https://doi.org/10.1016/j.tins.2009.05.001
Bolaños JP (2016) Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 139:115–125. https://doi.org/10.1111/jnc.13486
Gonçalves C-A, Rodrigues L, Bobermin LD et al (2018) Glycolysis-Derived compounds from astrocytes that modulate synaptic communication. Front Neurosci 12:1035. https://doi.org/10.3389/fnins.2018.01035
Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620. https://doi.org/10.1016/j.it.2016.06.006
Jensen CJ, Massie A, De Keyser J (2013) Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol 8:824–839. https://doi.org/10.1007/s11481-013-9480-6
Bobermin LD, Roppa RHA, Quincozes-Santos A (2019) Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim Biophys Acta Mol Basis Dis 1865:634–647. https://doi.org/10.1016/j.bbadis.2019.01.004
Gorina R, Font-Nieves M, Márquez-Kisinousky L et al (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59:242–255. https://doi.org/10.1002/glia.21094
Palmer AL, Ousman SS (2018) Astrocytes and aging Front Aging Neurosci 10:337. https://doi.org/10.3389/fnagi.2018.00337
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS ONE 8:e60282. https://doi.org/10.1371/journal.pone.0060282
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54:2969–2985. https://doi.org/10.1007/s12035-016-9880-8
Santos CL, Roppa PHA, Truccolo P et al (2018) Age-dependent neurochemical remodeling of hypothalamic astrocytes. Mol Neurobiol 55:5565–5579. https://doi.org/10.1007/s12035-017-0786-x
Longoni A, Bellaver B, Bobermin LD et al (2018) Homocysteine induces glial reactivity in adult rat astrocyte cultures. Mol Neurobiol 55:1966–1976. https://doi.org/10.1007/s12035-017-0463-0
Santos CL, Bobermin LD, Souza DO, Quincozes-Santos A (2018) Leptin stimulates the release of pro-inflammatory cytokines in hypothalamic astrocyte cultures from adult and aged rats. Metab Brain Dis 33:2059–2063. https://doi.org/10.1007/s11011-018-0311-6
Wyss-Coray T (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539:180–186. https://doi.org/10.1038/nature20411
Davinelli S, Maes M, Corbi G et al (2016) Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges. Immun Ageing 13:16. https://doi.org/10.1186/s12979-016-0070-3
Franceschi C (2007) Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65:S173-176. https://doi.org/10.1111/j.1753-4887.2007.tb00358.x
Quincozes-Santos A, Gottfried C (2011) Resveratrol modulates astroglial functions: neuroprotective hypothesis: resveratrol modulates astroglial functions. Ann N Y Acad Sci 1215:72–78. https://doi.org/10.1111/j.1749-6632.2010.05857.x
Quincozes-Santos A, Bobermin LD, Latini A et al (2013) Resveratrol Protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 8:e64372. https://doi.org/10.1371/journal.pone.0064372
Bellaver B, Bobermin LD, Souza DG et al (2016) Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim Biophys Acta BBA - Mol Basis Dis 1862:1827–1838. https://doi.org/10.1016/j.bbadis.2016.06.018
Rosa PM, Martins LAM, Souza DO, Quincozes-Santos A (2018) Glioprotective effect of resveratrol: an emerging therapeutic role for oligodendroglial cells. Mol Neurobiol 55:2967–2978. https://doi.org/10.1007/s12035-017-0510-x
Bonkowski MS, Sinclair DA (2016) Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol 17:679–690. https://doi.org/10.1038/nrm.2016.93
Bhullar KS, Hubbard BP (2015) Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta BBA - Mol Basis Dis 1852:1209–1218. https://doi.org/10.1016/j.bbadis.2015.01.012
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506. https://doi.org/10.1038/nrd2060
Price NL, Gomes AP, Ling AJY et al (2012) SIRT1 Is Required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Hwang J, Yao H, Caito S et al (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
Lee S-H, Lee J-H, Lee H-Y, Min K-J (2019) Sirtuin signaling in cellular senescence and aging. BMB Rep 52:24–34. https://doi.org/10.5483/BMBRep.2019.52.1.290
Sarubbo F, Esteban S, Miralles A, Moranta D (2018) Effects of resveratrol and other polyphenols on Sirt1: relevance to brain function during aging.Curr Neuropharmacol 16https://doi.org/10.2174/1570159X15666170703113212
Bellaver B, Souza DG, Bobermin LD et al (2015) Resveratrol Protects hippocampal astrocytes against lps-induced neurotoxicity through HO-1, p38 and erk pathways. Neurochem Res 40:1600–1608. https://doi.org/10.1007/s11064-015-1636-8
Bobermin LD, Quincozes-Santos A, Guerra MC et al (2012) Resveratrol prevents ammonia toxicity in astroglial cells. PLoS ONE 7:e52164. https://doi.org/10.1371/journal.pone.0052164
Allen EN, Potdar S, Tapias V et al (2018) Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J Nutr Biochem 54:77–86. https://doi.org/10.1016/j.jnutbio.2017.10.015
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol Vitro Int J Publ Assoc BIBRA 28:479–484. https://doi.org/10.1016/j.tiv.2014.01.006
Bobermin LD, Roppa RHA, Gonçalves C-A, Quincozes-Santos A (2020) ammonia-induced glial-inflammaging. Mol Neurobiol 57:3552–3567. https://doi.org/10.1007/s12035-020-01985-4
Guerra M, Tortorelli LS, Galland F et al (2011) Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats. J Neuroinflammation 8:128. https://doi.org/10.1186/1742-2094-8-128
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif 25:402–408. https://doi.org/10.1006/meth.2001.1262
Quincozes-Santos A, Nardin P, de Souza DF et al (2009) The janus face of resveratrol in astroglial cells. Neurotox Res 16:30–41. https://doi.org/10.1007/s12640-009-9042-0
Reers M, Smiley ST, Mottola-Hartshorn C et al (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417. https://doi.org/10.1016/0076-6879(95)60154-6
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. free radical and antioxidant protocols. Humana Press, New Jersey, pp 347–352
Seelig GF, Meister A (1985) Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol 113:379–390. https://doi.org/10.1016/s0076-6879(85)13050-8
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Cohen J, Torres C (2019) Astrocyte senescence: evidence and significance. Aging Cell 18:e12937. https://doi.org/10.1111/acel.12937
Clarke LE, Liddelow SA, Chakraborty C et al (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci 115:E1896–E1905. https://doi.org/10.1073/pnas.1800165115
Franceschi C, Garagnani P, Parini P et al (2018) Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590. https://doi.org/10.1038/s41574-018-0059-4
Calabrese V, Santoro A, Monti D et al (2018) Aging and Parkinson’s Disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med 115:80–91. https://doi.org/10.1016/j.freeradbiomed.2017.10.379
Souza DG, Bellaver B, Bobermin LD et al (2016) Anti-aging effects of guanosine in glial cells. Purinergic Signal 12:697–706. https://doi.org/10.1007/s11302-016-9533-4
Souza DG, Bellaver B, Raupp GS et al (2015) Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int 90:93–97. https://doi.org/10.1016/j.neuint.2015.07.016
Wyse AT, Siebert C, Bobermin LD et al (2020) Changes in inflammatory response, redox status and Na+, K+-ATPase activity in primary astrocyte cultures from female wistar rats subject to ovariectomy. Neurotox Res 37:445–454. https://doi.org/10.1007/s12640-019-00128-5
Kodali M, Parihar VK, Hattiangady B et al (2015) Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci Rep 5:8075. https://doi.org/10.1038/srep08075
Lalo U, Pankratov Y (2021) Astrocytes as perspective targets of exercise- and caloric restriction-mimetics. Neurochem Res. https://doi.org/10.1007/s11064-021-03277-2
Zhu X, Chen Z, Shen W et al (2021) Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther 6:245. https://doi.org/10.1038/s41392-021-00646-9
Ahmed A, Williams B, Hannigan G (2015) Transcriptional activation of inflammatory genes: mechanistic insight into selectivity and diversity. Biomolecules 5:3087–3111. https://doi.org/10.3390/biom5043087
Bettio LEB, Rajendran L, Gil-Mohapel J (2017) The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 79:66–86. https://doi.org/10.1016/j.neubiorev.2017.04.030
Fan X, Wheatley EG, Villeda SA (2017) Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci 40:251–272. https://doi.org/10.1146/annurev-neuro-072116-031357
van Horssen J, van Schaik P, Witte M (2019) Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci Lett 710:132931. https://doi.org/10.1016/j.neulet.2017.06.050
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503. https://doi.org/10.1016/j.redox.2018.01.008
López-Navarro ME, Jarquín-Martínez M, Sánchez-Labastida LA et al (2020) Decoding aging: understanding the complex relationship among aging, free radicals, and GSH. Oxid Med Cell Longev 2020:1–11. https://doi.org/10.1155/2020/3970860
Arús BA, Souza DG, Bellaver B et al (2017) Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol Cell Biochem 428:67–77. https://doi.org/10.1007/s11010-016-2917-5
Potier B, Billard J-M, Rivière S et al (2010) Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats: synaptic effects of reduced glutamate uptake in the aged rat hippocampus. Aging Cell 9:722–735. https://doi.org/10.1111/j.1474-9726.2010.00593.x
Todd AC, Hardingham GE (2020) The regulation of astrocytic glutamate transporters in health and neurodegenerative diseases. Int J Mol Sci 21:9607. https://doi.org/10.3390/ijms21249607
Barnes JR, Mukherjee B, Rogers BC et al (2020) The relationship between glutamate dynamics and activity-dependent synaptic plasticity. J Neurosci 40:2793–2807. https://doi.org/10.1523/JNEUROSCI.1655-19.2020
Zhang Y, Sloan SA, Clarke LE et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. https://doi.org/10.1016/j.neuron.2015.11.013
Tian G, Lai L, Guo H et al (2007) Translational control of glial glutamate transporter EAAT2 expression. J Biol Chem 282:1727–1737. https://doi.org/10.1074/jbc.M609822200
Ryan RM, Ingram SL, Scimemi A (2021) Regulation of glutamate, GABA and dopamine transporter uptake, surface mobility and expression. Front Cell Neurosci 15:670346. https://doi.org/10.3389/fncel.2021.670346
Piao C, Ranaivo HR, Rusie A et al (2015) Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway. Exp Neurol 273:288–300. https://doi.org/10.1016/j.expneurol.2015.09.009
Miralles VJ, Martínez-López I, Zaragozá R et al (2001) Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res 922:21–29. https://doi.org/10.1016/s0006-8993(01)03124-9
Hertz L (2003) Astrocytic amino acid metabolism under control conditions and during oxygen and/or glucose deprivation. Neurochem Res 28:243–258. https://doi.org/10.1023/A:1022377100379
Tong L, Balazs R, Soiampornkul R et al (2008) Interleukin-1β impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging 29:1380–1393. https://doi.org/10.1016/j.neurobiolaging.2007.02.027
Lima Giacobbo B, Doorduin J, Klein HC et al (2019) Brain-Derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56:3295–3312. https://doi.org/10.1007/s12035-018-1283-6
Budni J, Bellettini-Santos T, Mina F, et al (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6:331. https://doi.org/10.14336/AD.2015.0825
Sakata Y, Zhuang H, Kwansa H et al (2010) Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol 224:325–329. https://doi.org/10.1016/j.expneurol.2010.03.032
Liddell J (2017) Are Astrocytes the predominant cell type for activation of nrf2 in Aging and neurodegeneration? Antioxidants 6:65. https://doi.org/10.3390/antiox6030065
Ahmed SMU, Luo L, Namani A et al (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta BBA - Mol Basis Dis 1863:585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
Kauppinen A, Suuronen T, Ojala J et al (2013) Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25:1939–1948. https://doi.org/10.1016/j.cellsig.2013.06.007
Bellaver B, Dos Santos JP, Leffa DT et al (2018) Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol Neurobiol 55:2685–2695. https://doi.org/10.1007/s12035-017-0526-2
Cian RE, López-Posadas R, Drago SR et al (2012) A Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-κB, and p38 and JNK dependent mechanism. Food Chem 134:1982–1990. https://doi.org/10.1016/j.foodchem.2012.03.134
Xu X, Guo Y, Zhao J et al (2017) Punicalagin, a PTP1B inhibitor, induces M2c phenotype polarization via up-regulation of HO-1 in murine macrophages. Free Radic Biol Med 110:408–420. https://doi.org/10.1016/j.freeradbiomed.2017.06.014
Rodgers KR, Lin Y, Langan TJ et al (2020) Innate Immune functions of astrocytes are dependent upon tumor necrosis factor-alpha. Sci Rep 10:7047. https://doi.org/10.1038/s41598-020-63766-2
Villarreal A, Seoane R, González Torres A et al (2014) S100B protein activates a RAGE-dependent autocrine loop in astrocytes: implications for its role in the propagation of reactive gliosis. J Neurochem 131:190–205. https://doi.org/10.1111/jnc.12790
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Universidade Federal do Rio Grande do Sul and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Author information
Authors and Affiliations
Contributions
AQS and LDB conceptualized the study. LDB, RRSA, FBW, LSM, and LM performed the experiments. LDB and AQS performed statistical analysis and written the original draft of the manuscript. AQS, ATSW, and CAG provided resources and materials/chemicals. All authors revised, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and Brazilian Society for Neuroscience and Behavior recommendations for animal care. The experimental protocols were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number: 21215).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Figure S1.
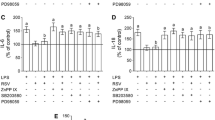
Effects of HO-1 inhibitor on cytokine release in aged astrocytes. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM) and HO-1 inhibitor ZnPP IX (10 μM). The extracellular levels of TNF-α (A), IL-1β (B), IL-6 (C), MCP-1 (D), and IL-10 (E) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol. (PNG 14239 kb)

Figure S2.
Effects of HO-1 and p38 MAPK inhibitors on glutamate uptake in aged astrocytes. Primary hippocampal astrocyte cultures from aged rats were incubated with LPS (1 μg/ml) for 24 h in the presence or absence of resveratrol (10 μM) and HO-1 inhibitor ZnPP IX (10 μM) or p38 MAPK inhibitor SB203580 (10 μM), A and B, respectively. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s test (n = 6 independent cultures and, at least, duplicate of treatments). Values of P < 0.05 were considered significant. a refers to statistically significant differences from the control conditions; b refers to statistically significant differences from LPS challenge. RSV, resveratrol. (PNG 8928 kb)
Rights and permissions
About this article
Cite this article
Bobermin, L.D., de Souza Almeida, R.R., Weber, F.B. et al. Lipopolysaccharide Induces Gliotoxicity in Hippocampal Astrocytes from Aged Rats: Insights About the Glioprotective Roles of Resveratrol. Mol Neurobiol 59, 1419–1439 (2022). https://doi.org/10.1007/s12035-021-02664-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02664-8