Abstract
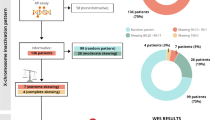
Intellectual disability (ID) affects 30% more males than females. This sex bias can be attributed to the enrichment of genes on the X chromosome playing essential roles in the central nervous system and their hemizygous state on males. Moreover, as a result of X chromosome inactivation (XCI), most genes on one of the X chromosomes in female somatic cells are epigenetically silenced, so that females carrying X-linked variants are not expected to be so severely affected as males. Consequently, the knowledge about X-linked ID (XLID) in females is still scarce. Herein, we used extreme XCI skewing (≥ 90%) to predict X-linked variants in females with idiopathic ID. XCI profiles from 53 probands were estimated from blood and buccal mucosa through a methylation-sensitive AR/RP2 assay. DNA samples with extreme XCI skewing were then submitted to array-comparative genomic hybridization and whole-exome sequencing. Seven females (13.2%) exhibited extreme XCI skewing, a percentage significantly higher than expected for healthy females in our population. XLID-potentially related variants were identified in five patients with extreme XCI skewing, including one pathogenic rstructural rearrangement [der(X) chromosome from a t(X;2)] and four single nucleotide variants in NLGN4X, HDAC8, TAF1, and USP9X genes, two of which affecting XCI escape genes. XCI skewing showed to be an outstanding approach for the characterization of molecular mechanisms underlying XLID in females. Beyond expanding the spectrum of variants/phenotypes associated with ID, our results pointed to compensatory biological pathways underlying XCI and uncover new insights into the involvement of escape genes on XLID, impacting genetic counseling.
Similar content being viewed by others
References
Neri G, Schwartz CE, Lubs HA, Stevenson RE (2018) X-linked intellectual disability update 2017. Am J Med Genet Part A 176:1375–1388. https://doi.org/10.1002/ajmg.a.38710
Greenwood Genetic Center, X-linked intellectual disability, 2020, https://www.ggc.org/xlid-genetic-research/
Fieremans N, Van Esch H, Holvoet M et al (2016) Identification of intellectual disability genes in female patients with a skewed X-inactivation pattern. Hum Mutat 37:804–811. https://doi.org/10.1002/humu.23012
Carrel L, Brown CJ (2017) When the Lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Phil Trans R Soc A B372:20160355. https://doi.org/10.1098/rstb.2016.0355
Ørstavik KH (2009) X chromosome inactivation in clinical practice. Hum Genet 126:363–373. https://doi.org/10.1007/s00439-009-0670-5
Plenge RM, Stevenson RA, Lubs HA, Schwartz CE, Willard HF (2002) Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am J Hum Genet 71:168–173. https://doi.org/10.1086/341123
Fieremans N, Bauters M, Belet S, Verbeeck J, Jansen AC, Seneca S, Roelens F, de Baere E et al (2014) De novo MECP2 duplications in two females with intellectual disability and unfavorable complete skewed X-inactivation. Hum Genet 133:1359–1367. https://doi.org/10.1007/s00439-014-1469-6
McMahon A, Monk M (1983) X-chromosome activity in female mouse embryos heterozygous for Pgk-1 and Searle’s translocation, T(X; 16) 16H. Genet Res 41:69–83. https://doi.org/10.1017/s0016672300021078
Gupta N, Goel H, Phadke SR (2006) Unbalanced X; autosome translocation. Indian J Pediatr 73:840–842. https://doi.org/10.1007/BF02790399
Balaton BP, Cotton AM, Brown CJ (2015) Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biol Sex Differ 6:35. https://doi.org/10.1186/s13293-015-0053-7
Carrel L, Willard HF (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434:400–404. https://doi.org/10.1038/nature03479
Tukiainen T, Villani A-C, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L et al (2017) Landscape of X chromosome inactivation across human tissues. Nature 550:244–248. https://doi.org/10.1038/nature24265
Machado FB, Faria MA, Lovatel VL et al (2014) 5meCpG epigenetic marks neighboring a primate-conserved core promoter short tandem repeat indicate X-chromosome inactivation. PLoS One 9:e103714. https://doi.org/10.1371/journal.pone.0103714
Rosenberg C, Freitas ÉL, Uehara DT, Auricchio MTBM, Costa SS, Oiticica J, Silva AG, Krepischi AC et al (2016) Genomic copy number alterations in non-syndromic hearing loss. Clin Genet 89:473–477. https://doi.org/10.1111/cge.12683
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine 17:405–423. https://doi.org/10.1038/gim.2015.30
Li Q, Wang K (2017) InterVar: clinical interpretation of genetic variants by ACMG-AMP 2015 guideline. Am J Hum Genet 100:1–14. https://doi.org/10.1016/j.ajhg.2017.01.004
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, Massouras A (2018) VarSome: the human genomic variant search engine. Oxford Bioinformatics 35:1978–1980. https://doi.org/10.1093/bioinformatics/bty897
El-Gebali S, Mistry J, Bateman A et al (2019) The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. https://doi.org/10.1093/nar/gky995
Liu W, Xie Y, Ma J, Luo X, Nie P, Zuo Z, Lahrmann U, Zhao Q et al (2015) Sequence analysis IBS : an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31:3359–3361. https://doi.org/10.1093/bioinformatics/btv362
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Vissers LELM, Gilissen C, Veltman JA (2016) Genetic studies in intellectual disability and related disorders. Nat Rev Genet 17:9–18. https://doi.org/10.1038/nrg3999
Gonçalves TF, dos Santos JM, Gonçalves AP, Tassone F, Mendoza-Morales G, Ribeiro MG, Kahn E, Boy R et al (2016) Finding FMR1 mosaicism in fragile X syndrome. Expert Rev Mol Diagn 16:501–507. https://doi.org/10.1586/14737159.2016.1135739
Bittel DC, Theodoro MF, Kibiryeva N, Fischer W, Talebizadeh Z, Butler MG (2008) Comparison of X-chromosome inactivation patterns in multiple tissues from human females. J Med Genet 45:309–313. https://doi.org/10.1136/jmg.2007.055244
Cotton AM, Lam L, Affleck JG, Wilson IM, Peñaherrera MS, McFadden DE, Kobor MS, Lam WL et al (2011) Chromosome-wide DNA methylation analysis predicts human tissue-specific X inactivation. Hum Genet 130:187–201. https://doi.org/10.1007/s00439-011-1007-8
Sharp A, Robinson D, Jacobs P (2000) Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet 107:343–349. https://doi.org/10.1007/s004390000382
Borges BPCN (2018) Investigação de padrões de inativação do cromossomo X em mulheres saudáveis residentes no Estado do Rio de Janeiro. Universidade do Estado do Rio de Janeiro
Ziats CA, Schwartz CE, Gecz J et al (2020) X-linked intellectual disability: phenotypic expression in carrier females. Clin Genet 97:418–425. https://doi.org/10.1111/cge.13667
Busque L, Paquette Y, Provost S, Roy DC, Levine RL, Mollica L, Gary Gilliland D (2009) Skewing of X-inactivation ratios in blood cells of aging women is confirmed by independent methodologies. Blood 113:3472–3474. https://doi.org/10.1182/blood-2008-12-195677
Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G et al (2013) The genotype-tissue expression (GTEx) project. Nat Genet 45:580–585. https://doi.org/10.1038/ng.2653
Li JY, Daniels G, Wang J, Zhang X (2015) TBL1XR1 in physiological and pathological states. Am J Clin Exp Urol 3:13–23 http://www.ncbi.nlm.nih.gov/pubmed/26069883
Kruusvee V, Lyst MJ, Taylor C, Tarnauskaitė Ž, Bird AP, Cook AG (2017) Structure of the MeCP2–TBLR1 complex reveals a molecular basis for Rett syndrome and related disorders. PNAS 114:E3243–E3250. https://doi.org/10.1073/pnas.1700731114
Zaghlula M, Glaze DG, Enns GM, Potocki L, Schwabe AL, Suter B (2018) Current clinical evidence does not support a link between TBL1XR1 and Rett syndrome: description of one patient with Rett features and a novel mutation in TBL1XR1, and a review of TBL1XR1 phenotypes. Am J Med Genet Part A 176:1683–1687. https://doi.org/10.1002/ajmg.a.38689
Vaqueiro AC, de Oliveira CP, Cordoba MS, Versiani BR, de Carvalho CX, Alves Rodrigues PG, de Oliveira SF, Mazzeu JF et al (2018) Expanding the spectrum of TBL1XR1 deletion: report of a patient with brain and cardiac malformations. Eur J Med Genet 61:29–33. https://doi.org/10.1016/j.ejmg.2017.10.008
Armour CM, Smith A, Hartley T, Chardon JW, Sawyer S, Schwartzentruber J, Hennekam R, Majewski J et al (2016) Syndrome disintegration: exome sequencing reveals that Fitzsimmons syndrome is a co-occurrence of multiple events. Am J Med Genet Part A 170:1820–1825. https://doi.org/10.1002/ajmg.a.37684
O’Roak BJ, Vives L, Fu W et al (2012) Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338:1619–1622. https://doi.org/10.1126/science.1227764
O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A et al (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250. https://doi.org/10.1038/nature10989
Saitsu H, Tohyama J, Walsh T, Kato M, Kobayashi Y, Lee M, Tsurusaki Y, Miyake N et al (2014) A girl with West syndrome and autistic features harboring a de novo TBL1XR1 mutation. J Hum Genet 59:581–583. https://doi.org/10.1038/jhg.2014.71
Pons L, Cordier MP, Labalme A, Till M, Louvrier C, Schluth-Bolard C, Lesca G, Edery P et al (2015) A new syndrome of intellectual disability with dysmorphism due to TBL1XR1 deletion. Am J Med Genet Part A 167:164–168. https://doi.org/10.1002/ajmg.a.36759
Tabet A-C, Leroy C, Dupont C, Serrano E, Hernandez K, Gallard J, Pouvreau N, Gadisseux JF et al (2014) De novo deletion of TBL1XR1 in a child with non-specific developmental delay supports its implication in intellectual disability. Am J Med Genet Part A 164:2335–2337. https://doi.org/10.1002/ajmg.a.36619
Riehmer V, Erger F, Herkenrath P, Seland S, Jackels M, Wiater A, Heller R, Beck BB et al (2017) A heritable microduplication encompassing TBL1XR1 causes a genomic sister-disorder for the 3q26.32 microdeletion syndrome. Am J Med Genet Part A 173:2132–2138. https://doi.org/10.1002/ajmg.a.38285
Żylicz JJ, Bousard A, Žumer K et al (2019) The implication of early chromatin changes in X chromosome inactivation. Cell 176:182–197.e23. https://doi.org/10.1016/j.cell.2018.11.041
Zhang J, Kalkum M, Chait BT, Roeder RG (2002) The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell 9:611–623. https://doi.org/10.1016/s1097-2765(02)00468-9
Banerjee S, Riordan M, Bhat MA (2014) Genetic aspects of autism spectrum disorders: insights from animal models. Front Cell Neurosci 8:58. https://doi.org/10.3389/fncel.2014.00058
Landini M, Merelli I, Raggi M, Galluccio N, Ciceri F, Bonfanti A, Camposeo S, Massagli A et al (2016) Association analysis of noncoding variants in neuroligins 3 and 4x genes with autism spectrum disorder in an Italian cohort. Int J Mol Sci 17:1765. https://doi.org/10.3390/ijms17101765
Bouazzi H, Lesca G, Trujillo C, Alwasiyah MK, Munnich A (2015) Nonsyndromic X-linked intellectual deficiency in three brothers with a novel MED12 missense mutation [c.5922G>T (p.Glu1974His)]. Clin Case Rep 3:604–609. https://doi.org/10.1002/ccr3.301
Cukier HN, Dueker ND, Slifer SH, Lee JM, Whitehead PL, Lalanne E, Leyva N, Konidari I et al (2014) Exome sequencing of extended families with autism reveals genes shared across neurodevelopmental and neuropsychiatric disorders. Mol Autism 5:1. https://doi.org/10.1186/2040-2392-5-1
Kou Y, Betancur C, Xu H, Buxbaum JD, Ma’ayan A (2012) Network- and attribute-based classifiers can prioritize genes and pathways for autism spectrum disorders and intellectual disability. Am J Med Genet Part C 160:130–142. https://doi.org/10.1002/ajmg.c.31330
Murtaza M, Jolly LA, Gecz J, Wood SA (2015) La FAM fatale: USP9X in development and disease. Cell Mol Life Sci 72:2075–2089. https://doi.org/10.1007/s00018-015-1851-0
Johnson BV, Kumar R, Oishi S, Alexander S, Kasherman M, Vega MS, Ivancevic A, Gardner A et al (2019) Partial loss of USP9X function leads to a male neurodevelopmental and behavioral disorder converging on transforming growth factor β signaling. Biol Psychiatry 87:100–112. https://doi.org/10.1016/j.biopsych.2019.05.028
Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB (2013) Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet 9:e1003709. https://doi.org/10.1371/journal.pgen.1003709
Reijnders MRF, Zachariadis V, Latour B, Jolly L, Mancini GM, Pfundt R, Wu KM, van Ravenswaaij-Arts C et al (2016) De novo loss-of-function mutations in USP9X cause a female-specific recognizable syndrome with developmental delay and congenital malformations. Am J Hum Genet 98:373–381. https://doi.org/10.1016/j.ajhg.2015.12.015
Peeters SB, Cotton AM, Brown CJ (2014) Variable escape from X-chromosome inactivation: identifying factors that tip the scales towards expression. BioEssays 36:746–756. https://doi.org/10.1002/bies.201400032
Talebizadeh Z, Simon SD, Butler MG (2006) X chromosome gene expression in human tissues: male and female comparisons. Genomics 88:675–681. https://doi.org/10.1016/j.ygeno.2006.07.016
Yang C, Chapman AG, Kelsey AD, Minks J, Cotton AM, Brown CJ (2011) X-chromosome inactivation: molecular mechanisms from the human perspective. Hum Genet 130:175–185. https://doi.org/10.1007/s00439-011-0994-9
Zweier C, Kraus C, Brueton L, Cole T, Degenhardt F, Engels H, Gillessen-Kaesbach G, Graul-Neumann L et al (2013) A new face of Borjeson–Forssman–Lehmann syndrome? De novo mutations in PHF6 in seven females with a distinct phenotype. J Med Genet 50:838–847. https://doi.org/10.1136/jmedgenet-2013-101918
Snijders Blok L, Madsen E, Juusola J, Gilissen C, Baralle D, Reijnders MR, Venselaar H, Helsmoortel C et al (2015) Mutations in DDX3X are a common cause of unexplained intellectual disability with gender-specific effects on Wnt signaling. Am J Hum Genet 97:343–352. https://doi.org/10.1016/j.ajhg.2015.07.004
Deardorff MA, Bando M, Nakato R, Watrin E, Itoh T, Minamino M, Saitoh K, Komata M et al (2012) HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489:313–317. https://doi.org/10.1038/nature11316
Kaiser FJ, Ansari M, Braunholz D, Concepción Gil-Rodríguez M, Decroos C, Wilde JJ, Fincher CT, Kaur M et al (2014) Loss-of-function HDAC8 mutations cause a phenotypic spectrum of Cornelia de Lange syndrome-like features, ocular hypertelorism, large fontanelle and X-linked inheritance. Hum Mol Genet 23:2888–2900. https://doi.org/10.1093/hmg/ddu002
Feng L, Zhou D, Zhang Z, Liu Y, Yang Y (2014) Exome sequencing identifies a de novo mutation in HDAC8 associated with Cornelia de Lange syndrome. J Hum Genet 59:536–539. https://doi.org/10.1038/jhg.2014.60
Harakalova M, van den Boogaard M-J, Sinke R, van Lieshout S, van Tuil MC, Duran K, Renkens I, Terhal PA et al (2012) X-exome sequencing identifies a HDAC8 variant in a large pedigree with X-linked intellectual disability, truncal obesity, gynaecomastia, hypogonadism and unusual face. J Med Genet 49:539–543. https://doi.org/10.1136/jmedgenet-2012-100921
Krawczynska N, Wierzba J, Wasag B (2019) Genetic mosaicism in a group of patients with Cornelia de Lange syndrome. Front Pediatr 7:203. https://doi.org/10.3389/fped.2019.00203
Helgeson M, Keller-Ramey J, Knight Johnson A, Lee JA, Magner DB, Deml B, Deml J, Hu YY et al (2018) Molecular characterization of HDAC8 deletions in individuals with atypical Cornelia de Lange syndrome. J Hum Genet 63:349–356. https://doi.org/10.1038/s10038-017-0387-6
Dowsett L, Porras AR, Kruszka P, Davis B, Hu T, Honey E, Badoe E, Thong MK et al (2019) Cornelia de Lange syndrome in diverse populations. Am J Med Genet A 179:150–158. https://doi.org/10.1002/ajmg.a.61033
O’Rawe JA, Wu Y, Dörfel MJ, Rope AF, Au PYB, Parboosingh JS, Moon S, Kousi M et al (2015) TAF1 variants are associated with dysmorphic features, intellectual disability, and neurological manifestations. Am J Hum Genet 97:922–932. https://doi.org/10.1016/j.ajhg.2015.11.005
Gudmundsson S, Wilbe M, Filipek-Górniok B, Molin AM, Ekvall S, Johansson J, Allalou A, Gylje H et al (2019) TAF1, associated with intellectual disability in humans, is essential for embryogenesis and regulates neurodevelopmental processes in zebrafish. Sci Rep 9:10730. https://doi.org/10.1038/s41598-019-46632-8
Janakiraman U, Yu J, Moutal A, Chinnasamy D, Boinon L, Batchelor SN, Anandhan A, Khanna R et al (2019) TAF1-gene editing alters the morphology and function of the cerebellum and cerebral cortex. Neurobiol Dis 132:104539. https://doi.org/10.1016/j.nbd.2019.104539
Domingo A, Amar D, Grütz K, Lee LV, Rosales R, Brüggemann N, Jamora RD, Cutiongco-dela Paz E et al (2016) Evidence of TAF1 dysfunction in peripheral models of X-linked dystonia-parkinsonism. Cell Mol Life Sci 73:3205–3215. https://doi.org/10.1007/s00018-016-2159-4
Hurst SE, Liktor-Busa E, Moutal A, Parker S, Rice S, Szelinger S, Senner G, Hammer MF et al (2018) A novel variant in TAF1 affects gene expression and is associated with X-linked TAF1 intellectual disability syndrome. Neuronal Signaling 2:NS20180141. https://doi.org/10.1042/NS20180141
Sans N, Ezan J, Moreau MM, Montcouquiol M (2016) Planar cell polarity gene mutations in autism spectrum disorder, intellectual disabilities, and related deletion/duplication syndromes. In neuronal and synaptic dysfunction in autism spectrum disorder and intellectual disability. Elsevier:189–219. https://doi.org/10.1016/B978-0-12-800109-7.00013-3
White J, Mazzeu JF, Hoischen A, Jhangiani SN, Gambin T, Alcino MC, Penney S, Saraiva JM et al (2015) DVL1 frameshift mutations clustering in the penultimate exon cause autosomal-dominant Robinow syndrome. Am J Hum Genet 96:612–622. https://doi.org/10.1016/j.ajhg.2015.02.015
Long JM, LaPorte P, Paylor R, Wynshaw-Boris A (2004) Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav 3:51–62. https://doi.org/10.1046/j.1601-183x.2003.00045.x
Santos-Rebouças CB, Boy R, Vianna EQ, Gonçalves AP, Piergiorge RM, Abdala BB, dos Santos JM, Calassara V et al (2020) Skewed X-chromosome inactivation and compensatory upregulation of escape genes precludes major clinical symptoms in a female with a large Xq deletion. Front Genet 11:101. https://doi.org/10.3389/fgene.2020.00101
Carrette LLG, Wang C-Y, Wei C, Press W, Ma W, Kelleher RJ III, Lee JT (2018) A mixed modality approach towards Xi reactivation for Rett syndrome and other X-linked disorders. PNAS 115:E668–E675. https://doi.org/10.1073/pnas.1715124115
Jiang J, Jing Y, Cost GJ, Chiang JC, Kolpa HJ, Cotton AM, Carone DM, Carone BR et al (2013) Translating dosage compensation to trisomy 21. Nature 500:296–300. https://doi.org/10.1038/nature12394
Acknowledgments
The authors thank the studied families for their kind cooperation and Genome Facility Platform from the National Cancer Institute of Brazil.
Funding
This work was supported by funds from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa do Estado de São Paulo (CEPID - FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, and Centro de Produção da Universidade do Estado do Rio de Janeiro (CEPUERJ).
Author information
Authors and Affiliations
Contributions
Conception and design: C.B.S.R.
Acquisition of data: C.B.S.R, E.Q.V., R.M.P., A.P.G., J.M.S., V.C., A.C.V.K., C.R., F.B.M., E.M.A.
Clinical evaluation: R.T.B.S., S.R.S., M.G.R.
Analysis and interpretation: C.B.S.R, E.Q.V., R.M.P., A.C.V.K., C.R., M.M.G.P.
Manuscript drafting: C.B.S.R, E.Q.V., R.M.P, R.T.B.S., S.R.S., M.G.R.
Obtained funding: C.B.S.R, M.M.G.P., A.C.V.K., C.R.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Web Resources
The URLs for data presented herein are as follows:
ClinVar, 2020, https://www.ncbi.nlm.nih.gov/clinvar/
Database of Genomic Variants - DGV, 2020, http://projects.tcag.ca/variation/
Decipher v9.28, 2020, https://decipher.sanger.ac.uk/
Ensembl Genome Browser, 2020, http://www.ensembl.org/index.html
ExAC Browser, 2020, http://exac.broadinstitute.org/
Geneimprint, 2020, http://www.geneimprint.com/site/genes-by-species
GnomAD, 2020, https://gnomad.broadinstitute.org/
Mutation Taster, 2020, http://www.mutationtaster.org
OMIM, 2020, http://www.omim.org/
Pfam 32.0 platform, 2020, http://pfam.sanger.ac.uk/
RefSeq, 2020, http://www.ncbi.nlm.nih.gov/RefSeq
RepeatMasker program, 2020, http://repeatmasker.genome.washington.edu
UCSC Genome Browser (Hg19), 2020, http://genome.ucsc.edu
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PPTX 24481 kb)
Supplemental Table 2
In silico pathogenicity predictions and conservation data for the sequence variants found in females with extreme XCI skewing subjected to WES (excel file). (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Vianna, E.Q., Piergiorge, R.M., Gonçalves, A.P. et al. Understanding the Landscape of X-linked Variants Causing Intellectual Disability in Females Through Extreme X Chromosome Inactivation Skewing. Mol Neurobiol 57, 3671–3684 (2020). https://doi.org/10.1007/s12035-020-01981-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01981-8