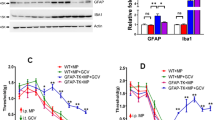
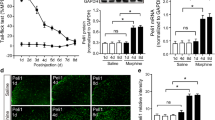
Abstract
The development of analgesic tolerance to opioids is an important limitation in the management of chronic pain. Spinal cord glial cell activation appears to play a pivotal role in the development and maintenance of opioid tolerance, indicating the presence of an opioid-induced neuronal–glial interaction; however, how opioids drive this cross-talk is still elusive. In search of treatments to attenuate morphine analgesic tolerance, our research focused on the role of Notch signaling pathway, one of the most important mechanisms of cell-to-cell interactions, in the spinal dorsal horn after morphine repeated exposure and whether Notch inhibition attenuates morphine analgesic tolerance. Double immunofluorescence experiments on spinal sections from morphine-tolerant mice showed a neuronal localization of Notch-1 receptor whereas the Notch ligand Jagged was localized on neighboring astrocytes. Morphine-induced μ opioid receptor (MOR) stimulation triggered Notch-1 signaling activation and this event was mediated by astrocyte JNK activation. Notch-1 activation selectively reduced the expression of histone deacetylase (HDAC)-1, resulting in an overphosphorylation of PKC and ERK, kinases involved in MOR phosphorylation and internalization after repeated morphine exposure. Notch-1 signaling inhibition, through intrathecal administration of the γ-secretase inhibitor, DAPT, counteracted PKC and ERK overphosphorylation, MOR internalization, and analgesic tolerance. Conversely, the HDAC-1 inhibitor, LG325, further aggravated MOR internalization, PKC overphosphorylation, and analgesic tolerance.
Our findings implicate the MOR-triggered Notch-1 signaling in promoting MOR internalization and morphine analgesic tolerance by epigenetic regulation mechanisms. These data suggest that Notch-1 inhibitors could represent an innovative therapeutic perspective for the management of opioid tolerance in chronic pain therapy.








Similar content being viewed by others
Abbreviations
- acH3K9:
-
Histone H3 acetyl K9
- DNMT:
-
DNA methyltransferase
- ERK:
-
Extracellular signal-regulated kinase
- GFAP:
-
Glial fibrillary acidic protein
- HDAC:
-
Histone deacetylase
- i.p.:
-
Intraperitoneal
- i.t.:
-
Intrathecal
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated kinase
- MOR:
-
μ Opioid receptor
- NeuN:
-
Neuronal-specific nuclear protein
- NEXT:
-
Notch extracellular truncation
- NICD:
-
Notch intracellular domain
- PKC:
-
Protein kinase C
- s.c.:
-
Subcutaneous
References
Arttamangkul S, Heinz DA, Bunzow JR, Song X, Williams JT (2018) Cellular tolerance at the m-opioid receptor is phosphorylation dependent. eLife 7:e34989
Bai G, Wei D, Zou S, Ren K, Dubner R (2010) Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain 6:51
Borggrefe T, Oswald F (2009) The notch signaling pathway: transcriptional regulation at notch target genes. Cell Mol Life Sci 66:1631–1646
Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689
Cai Y, Kong H, Pan YB, Jiang L, Pan XX, Hu L, Qian YN, Jiang CY et al (2016) Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J Neuroinflammation 13:53
Capasso KE, Manners MT, Quershi RA, Tian Y, Gao R, Hu H, Barrett JE, Sacan A et al (2015) Effect of histone deacetylase inhibitor JNJ-26481585 in pain. J Mol Neurosci 55:570–578
Charan J, Kantharia ND (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4:303–306
Chen Y, Geis C, Sommer C (2008) Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci 28:5836–5845
Chen ML, Cao H, Chu YX, Cheng LZ, Liang LL, Zhang YQ, Zhao ZQ (2012) Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: multiple glial–neuronal dialogues in the rat spinal cord. J Pain 13:945–958
Cherng CH, Lee KC, Chien CC, Chou KY, Cheng YC, Hsin ST, Lee SO, Shen CH et al (2014) Baicalin ameliorates neuropathic pain by suppressing HDAC1 expression in the spinal cord of spinal nerve ligation rats. J Formos Med Assoc 113:513–520
Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD et al (2017) Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 23:164–173
Denk F, Huang W, Sidders B, Bithell A, Crow M, Grist J, Sharma S, Ziemek D et al (2013) HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain 154:1668–1679
Eidson LN, Murphy AZ (2013) Persistent peripheral inflammation attenuates morphine-induced periaqueductal gray glial cell activation and analgesic tolerance in the male rat. J Pain 14:393–404
Fryer CJ, White JB, Jones KA (2004) Mastermind recruits CycC:CDK8 to phosphorylate the notch ICD and coordinate activation with turnover. Mol Cell 16:509–520
Galeotti N, Farzad M, Bianchi E, Ghelardini C (2014) PKC-mediated potentiation of morphine analgesia by St. John’s wort in rodents and humans. J Pharmacol Sci 124:409–417
Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KD, Devi LA (2016) G protein-coupled receptor heteromers. Annu Rev Pharmacol Toxicol 56:403–425
Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85:395–404
Grandbarbe L, Michelucci A, Heurtaux T, Hemmer K, Morga E, Heuschling P (2007) Notch signaling modulates the activation of microglial cells. Glia 55:1519–1530
Guo RX, Zhang M, Liu W, Zhao CM, Cui Y, Wang CH, Feng JQ, Chen PX (2009) NMDA receptors are involved in upstream of the spinal JNK activation in morphine antinociceptive tolerance. Neurosci Lett 467:95–99
Hameed H, Hameed M, Christo PJ (2010) The effect of morphine on glial cells as a potential therapeutic target for pharmacological development of analgesic drugs. Curr Pain Headache Rep 14:96–104
Horvath RJ, Romero-Sandoval EA, De Leo JA (2010) Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain 150:401–413
Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA (2010) Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience 169:843–854
Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, Tateya T, Kang YJ et al (2008) Integrated regulation of toll-like receptor responses by notch and interferon-gamma pathways. Immunity 29:691–703
Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K (2008) Overcoming failure to repair demyelination in EAE: gamma-secretase inhibition of notch signaling. J Neurol Sci 265:5–11
Kami K, Taguchi S, Tajima F, Senba E (2016) Histone acetylation in microglia contributes to exercise-induced hypoalgesia in neuropathic pain model mice. J Pain 17:588–599
Kao SC, Zhao X, Lee CY, Atianjoh FE, Gauda EB, Yaster M, Tao YX (2012) Absence of μ opioid receptor mRNA expression in astrocytes and microglia of rat spinal cord. NeuroReport 23:378–384
Koch T, Höllt V (2008) Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther 117:199–206
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Liang DY, Li X, Clark JD (2013) Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 14:36–47
Lin KT, Wang YW, Chen CT, Ho CM, Su WH, Jou YS (2012) HDAC inhibitors augmented cell migration and metastasis through induction of PKCs leading to identification of low toxicity modalities for combination cancer therapy. Clin Cancer Res 18:4691–4701
Louvi A, Artavanis-Tsakonas S (2006) Notch signalling in vertebrate neural development. Nat Rev Neurosci 7:93–102
Lubman OY, Ilagan MX, Kopan R, Barrick D (2007) Quantitative dissection of the notch:CSL interaction: insights into the notch-mediated transcriptional switch. J Mol Biol 365:577–589
Marcus DJ, Zee M, Hughes A, Yuill MB, Hohmann AG, Mackie K et al (2015) Tolerance to the antinociceptive effects of chronic morphine requires c-Jun N-terminal kinase. Mol Pain 11:34
Matsushita Y, Ueda H (2009) Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. Neuroreport 20:63–68
McGrath JC, Lilley E (2015) Implementing guidelines on reporting research using animals (ARRIVE etc.) new requirements for publication in BJP. Br J Pharmacol 172:3189–3193
Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A, Borea PA (2013) Morphine mediates a proinflammatory phenotype via mu-opioid receptor–PKCvarepsilon-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol 86:487–496
Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B (2009) Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun 23:75–84
Penas C, Navarro X (2018) Epigenetic modifications associated to neuroinflammation and neuropathic pain after neural trauma. Front Cell Neurosci 12:158
Pierfelice T, Alberi L, Gaiano N (2011) Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69:840–855
Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA (2003) Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain 104:655–664
Regan PM, Dave RS, Datta PK, Khalli K (2012) Epigenetics of m-opioid receptors: intersection with HIV-1 infection of the central nervous system. J Cell Physiol 227:2832–2841
Sanna MD, Ghelardini C, Galeotti N (2014) Regionally selective activation of ERK and JNK in morphine paradoxical hyperalgesia: a step toward improving opioid pain therapy. Neuropharmacology 86:67–77
Sanna MD, Ghelardini C, Galeotti N (2015) Activation of JNK pathway in spinal astrocytes contributes to acute ultra-low dose morphine thermal hyperalgesia. Pain 156:1265–1275
Sanna MD, Guandalini L, Romanelli MN, Galeotti N (2017) The new HDAC1 inhibitor LG325 ameliorates neuropathic pain in a mouse model. Pharmacol Biochem Behav 160:70–75
Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL (2007) Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain 127:129–139
Song P, Zhao ZQ (2001) The involvement of glial cells in the development of morphine tolerance. Neurosci Res 39:281–286
Sun Y-Y, Li L, Liu X-H, Gu N, Dong H-L, Xiong L (2012) The spinal notch signaling pathway plays a pivotal role in the development of neuropathic pain. Mol Brain 5:23
Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T (2001) Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron 29:45–55
Wang W (2011) Notch signaling and notch signaling modifiers. Int J Biochem Cell Biol 43:1550–1562
Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: Novel counter-regulators of opioid analgesia. Trends Neurosci 28:661–669
Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR (2011) Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc 110:487–494
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ et al (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254
Wongchana W, Lawlor RG, Osborne BA, Palaga T (2015) Impact of Notch1 deletion in macrophages on proinflammatory cytokine production and the outcome of experimental autoimmune encephalomyelitis. J Immunol 195:5337–5346
Xie K, Qiao F, Sun Y, Wang G, Hou L (2015) Notch signaling activation is critical to the development of neuropathic pain. BMC Anesthesiol 15:41
Yuill MB, Zee ML, Marcus D, Morgan DJ (2016) Tolerance to the antinociceptive and hypothermic effects of morphine is mediated by multiple isoforms of c-Jun N-terminal kinase. NeuroReport 27:392–396
Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ (2011) Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 17:1448–1455
Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR et al (2006) A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 26:3551–3560
Acknowledgements
This work was supported by grants from the University of Florence.
Author information
Authors and Affiliations
Contributions
N.G. designed the study and wrote the protocol. M.D.S., V.B. performed the experiments. N.G. managed the literature searches and analyses. N.G., M.D.S. undertook the statistical analysis. N.G. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanna, M.D., Borgonetti, V. & Galeotti, N. μ Opioid Receptor-Triggered Notch-1 Activation Contributes to Morphine Tolerance: Role of Neuron–Glia Communication. Mol Neurobiol 57, 331–345 (2020). https://doi.org/10.1007/s12035-019-01706-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01706-6