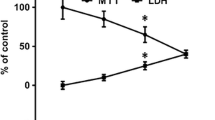
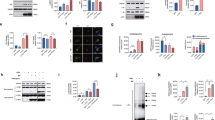
Abstract
Parkinson’s disease (PD) is one of the several neurodegenerative diseases where accumulation of aggregated proteins like α-synuclein occurs. Dysfunction in autophagy leading to this protein build-up and subsequent dopaminergic neurodegeneration may be one of the causes of PD. The mechanisms that impair autophagy remain poorly understood. 1-Methyl-4-phenylpiridium ion (MPP+) is a neurotoxin that induces experimental PD in vitro. Our studies have shown that glia maturation factor (GMF), a brain-localized inflammatory protein, induces dopaminergic neurodegeneration in PD and that suppression of GMF prevents MPP+-induced loss of dopaminergic neurons. In the present study, we demonstrate a molecular action of GMF on the autophagic machinery resulting in dopaminergic neuronal loss and propose GMF-mediated autophagic dysfunction as one of the contributing factors in PD progression. Using dopaminergic N27 neurons, primary neurons from wild type (WT), and GMF-deficient (GMF-KO) mice, we show that GMF and MPP+ enhanced expression of MAPKs increased the mammalian target of rapamycin (mTOR) activation and endoplasmic reticulum stress markers such as phospho-eukaryotic translation initiation factor 2 alpha kinase 3 (p-PERK) and inositol-requiring enzyme 1α (IRE1α). Further, GMF and MPP+ reduced Beclin 1, focal adhesion kinase (FAK) family-interacting protein of 200 kD (FIP200), and autophagy-related proteins (ATGs) 3, 5, 7, 16L, and 12. The combined results demonstrate that GMF affects autophagy through autophagosome formation with significantly reduced lysosomal-associated membrane protein 1/2, and the number of autophagic acidic vesicles. Using primary neurons, we show that MPP+ treatment leads to differential expression and localization of p62/sequestosome and in GMF-KO neurons, there was a marked increase in p62 staining implying autophagy deficiency with very little co-localization of α-synuclein and p62 as compared with WT neurons. Collectively, this study provides a bidirectional role for GMF in executing dopaminergic neuronal death mediated by autophagy that is relevant to PD.











Similar content being viewed by others
References
Khan MM, Kempuraj D, Zaheer S, Zaheer A (2014) Glia maturation factor deficiency suppresses 1-methyl-4-phenylpyridinium-induced oxidative stress in astrocytes. J Mol Neurosci 53(4):590–599. https://doi.org/10.1007/s12031-013-0225-z
Berry C, La Vecchia C, Nicotera P (2010) Paraquat and Parkinson’s disease. Cell Death Differ 17(7):1115–1125. https://doi.org/10.1038/cdd.2009.217
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29(13):1583–1590. https://doi.org/10.1002/mds.25945
Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 124(8):901–905. https://doi.org/10.1007/s00702-017-1686-y
de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, Manubens-Bertran JM, Alperovitch A et al (1997) Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J Neurol Neurosurg Psychiatry 62(1):10–15
Chua CE, Tang BL (2006) Alpha-synuclein and Parkinson’s disease: the first roadblock. J Cell Mol Med 10(4):837–846
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131(Pt 8):1969–1978. https://doi.org/10.1093/brain/awm318
Tan JM, Wong ES, Lim KL (2009) Protein misfolding and aggregation in Parkinson’s disease. Antioxid Redox Signal 11(9):2119–2134. https://doi.org/10.1089/ARS.2009.2490
Janda E, Isidoro C, Carresi C, Mollace V (2012) Defective autophagy in Parkinson’s disease: role of oxidative stress. Mol Neurobiol 46(3):639–661. https://doi.org/10.1007/s12035-012-8318-1
Laguna A, Schintu N, Nobre A, Alvarsson A, Volakakis N, Jacobsen JK, Gomez-Galan M, Sopova E et al (2015) Dopaminergic control of autophagic-lysosomal function implicates Lmx1b in Parkinson’s disease. Nat Neurosci 18(6):826–835. https://doi.org/10.1038/nn.4004
Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Mov Disord 26(6):1049–1055. https://doi.org/10.1002/mds.23732
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2(4):a009357. https://doi.org/10.1101/cshperspect.a009357
Mizushima N, Klionsky DJ (2007) Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 27:19–40. https://doi.org/10.1146/annurev.nutr.27.061406.093749
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873. https://doi.org/10.1101/gad.1599207
Kinghorn KJ, Asghari AM, Castillo-Quan JI (2017) The emerging role of autophagic-lysosomal dysfunction in Gaucher disease and Parkinson’s disease. Neural Regen Res 12(3):380–384. https://doi.org/10.4103/1673-5374.202934
Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, van de Berg WD (2017) Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener 12(1):11. https://doi.org/10.1186/s13024-017-0154-3
Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105(7):891–902
Hu ZY, Chen B, Zhang JP, Ma YY (2017) Up-regulation of autophagy-related gene 5 (ATG5) protects dopaminergic neurons in a zebrafish model of Parkinson’s disease. J Biol Chem 292(44):18062–18074. https://doi.org/10.1074/jbc.M116.764795
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231. https://doi.org/10.1128/MCB.01453-06
Takacs-Vellai K, Vellai T, Puoti A, Passannante M, Wicky C, Streit A, Kovacs AL, Muller F (2005) Inactivation of the autophagy gene bec-1 triggers apoptotic cell death in C. elegans. Curr Biol 15(16):1513–1517. https://doi.org/10.1016/j.cub.2005.07.035
Kon M, Cuervo AM (2010) Chaperone-mediated autophagy in health and disease. FEBS Lett 584(7):1399–1404. https://doi.org/10.1016/j.febslet.2009.12.025
Lim R, Zaheer A (1996) In vitro enhancement of p38 mitogen-activated protein kinase activity by phosphorylated glia maturation factor. J Biol Chem 271(38):22953–22956
Zaheer A, Lim R (1996) In vitro inhibition of MAP kinase (ERK1/ERK2) activity by phosphorylated glia maturation factor (GMF). Biochemistry 35(20):6283–6288. https://doi.org/10.1021/bi960034c
Zaheer A, Lim R (1997) Protein kinase A (PKA)- and protein kinase C-phosphorylated glia maturation factor promotes the catalytic activity of PKA. J Biol Chem 272(8):5183–5186
Zaheer S, Thangavel R, Sahu SK, Zaheer A (2011) Augmented expression of glia maturation factor in Alzheimer’s disease. Neuroscience 194:227–233. https://doi.org/10.1016/j.neuroscience.2011.07.069
Lim R, Zaheer A, Lane WS (1990) Complete amino acid sequence of bovine glia maturation factor beta. Proc Natl Acad Sci U S A 87(14):5233–5237
Lim R, Miller JF, Zaheer A (1989) Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A 86(10):3901–3905
Zaheer A, Fink BD, Lim R (1993) Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem 60(3):914–920
Zaheer A, Haas JT, Reyes C, Mathur SN, Yang B, Lim R (2006) GMF-knockout mice are unable to induce brain-derived neurotrophic factor after exercise. Neurochem Res 31(4):579–584. https://doi.org/10.1007/s11064-006-9049-3
Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A (2012) Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol 38(6):572–581. https://doi.org/10.1111/j.1365-2990.2011.01232.x
Lim R, Zaheer A, Khosravi H, Freeman JH Jr, Halverson HE, Wemmie JA, Yang B (2004) Impaired motor performance and learning in glia maturation factor-knockout mice. Brain Res 1024(1–2):225–232. https://doi.org/10.1016/j.brainres.2004.08.003
Zaheer A, Yang B, Cao X, Lim R (2004) Decreased copper-zinc superoxide dismutase activity and increased resistance to oxidative stress in glia maturation factor-null astrocytes. Neurochem Res 29(8):1473–1480
Kaimori JY, Takenaka M, Nakajima H, Hamano T, Horio M, Sugaya T, Ito T, Hori M et al (2003) Induction of glia maturation factor-beta in proximal tubular cells leads to vulnerability to oxidative injury through the p38 pathway and changes in antioxidant enzyme activities. J Biol Chem 278(35):33519–33527. https://doi.org/10.1074/jbc.M301552200
Baldwin RM, Garratt-Lalonde M, Parolin DA, Krzyzanowski PM, Andrade MA, Lorimer IA (2006) Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Ciota-mediated attenuation of p38 MAP kinase signaling. Oncogene 25(20):2909–2919. https://doi.org/10.1038/sj.onc.1209312
Zaheer S, Thangavel R, Wu Y, Khan MM, Kempuraj D, Zaheer A (2013) Enhanced expression of glia maturation factor correlates with glial activation in the brain of triple transgenic Alzheimer’s disease mice. Neurochem Res 38(1):218–225. https://doi.org/10.1007/s11064-012-0913-z
Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A (2013) Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. J NeuroImmune Pharmacol 8(3):643–650. https://doi.org/10.1007/s11481-013-9439-7
Zaheer A, Yorek MA, Lim R (2001) Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res 26(12):1293–1299
Khan MM, Zaheer S, Thangavel R, Patel M, Kempuraj D, Zaheer A (2015) Absence of glia maturation factor protects dopaminergic neurons and improves motor behavior in mouse model of parkinsonism. Neurochem Res 40(5):980–990. https://doi.org/10.1007/s11064-015-1553-x
Zupanska A, Dziembowska M, Ellert-Miklaszewska A, Gaweda-Walerych K, Kaminska B (2005) Cyclosporine a induces growth arrest or programmed cell death of human glioma cells. Neurochem Int 47(6):430–441. https://doi.org/10.1016/j.neuint.2005.05.010
Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG (2009) Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol 240(2):273–285. https://doi.org/10.1016/j.taap.2009.07.025
Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG (2011) Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol 256(3):227–240. https://doi.org/10.1016/j.taap.2011.07.018
Zaheer A, Mathur SN, Lim R (2002) Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun 294(2):238–244. https://doi.org/10.1016/S0006-291X(02)00467-9
Zawada WM, Banninger GP, Thornton J, Marriott B, Cantu D, Rachubinski AL, Das M, Griffin WS et al (2011) Generation of reactive oxygen species in 1-methyl-4-phenylpyridinium (MPP+) treated dopaminergic neurons occurs as an NADPH oxidase-dependent two-wave cascade. J Neuroinflammation 8:129. https://doi.org/10.1186/1742-2094-8-129
Ahmed ME, Tucker D, Dong Y, Lu Y, Zhao N, Wang R, Zhang Q (2016) Methylene blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience 336:39–48. https://doi.org/10.1016/j.neuroscience.2016.08.036
Gan SD, Patel KR (2013) Enzyme immunoassay and enzyme-linked immunosorbent assay. J Invest Dermatol 133(9):e12–e13. https://doi.org/10.1038/jid.2013.287
Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M (2010) Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 30(37):12535–12544. https://doi.org/10.1523/JNEUROSCI.1920-10.2010
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28(27):6926–6937. https://doi.org/10.1523/JNEUROSCI.0800-08.2008
Thome MP, Filippi-Chiela EC, Villodre ES, Migliavaca CB, Onzi GR, Felipe KB, Lenz G (2016) Ratiometric analysis of acridine orange staining in the study of acidic organelles and autophagy. J Cell Sci 129(24):4622–4632. https://doi.org/10.1242/jcs.195057
He Y, She H, Zhang T, Xu H, Cheng L, Yepes M, Zhao Y, Mao Z (2018) p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol 217(1):315–328. https://doi.org/10.1083/jcb.201701049
Mukherjee S, Dash S, Lohitesh K, Chowdhury R (2017) The dynamic role of autophagy and MAPK signaling in determining cell fate under cisplatin stress in osteosarcoma cells. PLoS One 12(6):e0179203. https://doi.org/10.1371/journal.pone.0179203
Crisan TO, Plantinga TS, van de Veerdonk FL, Farcas MF, Stoffels M, Kullberg BJ, van der Meer JW, Joosten LA et al (2011) Inflammasome-independent modulation of cytokine response by autophagy in human cells. PLoS One 6(4):e18666. https://doi.org/10.1371/journal.pone.0018666
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H et al (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368(7):610–622. https://doi.org/10.1056/NEJMoa1205158
Lang AE, Lozano AM (1998) Parkinson’s disease. Second of two parts. N Engl J Med 339(16):1130–1143. https://doi.org/10.1056/NEJM199810153391607
Pickrell Alicia M, Youle Richard J (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2):257–273. https://doi.org/10.1016/j.neuron.2014.12.007
Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2):257–273. https://doi.org/10.1016/j.neuron.2014.12.007
Lim R, Hicklin DJ, Ryken TC, Miller JF, Bosch EP (1988) Endogenous immunoreactive glia maturation factor-like molecule in cultured rat Schwann cells. Brain Res 468(2):277–284
Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R (2007) A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem 101(2):364–376. https://doi.org/10.1111/j.1471-4159.2006.04385.x
Asai K, Fujita K, Yamamoto M, Hotta T, Morikawa M, Kokubo M, Moriyama A, Kato T (1998) Isolation of novel human cDNA (hGMF-gamma) homologous to glia maturation factor-beta gene. Biochim Biophys Acta 1396(3):242–244
Inagaki M, Aoyama M, Sobue K, Yamamoto N, Morishima T, Moriyama A, Katsuya H, Asai K (2004) Sensitive immunoassays for human and rat GMFB and GMFG, tissue distribution and age-related changes. Biochim Biophys Acta 1670(3):208–216. https://doi.org/10.1016/j.bbagen.2003.12.006
Lim R, Zaheer A, Kraakevik JA, Darby CJ, Oberley LW (1998) Overexpression of glia maturation factor in C6 cells promotes differentiation and activates superoxide dismutase. Neurochem Res 23(11):1445–1451
Lim R, Zaheer A, Yorek MA, Darby CJ, Oberley LW (2000) Activation of nuclear factor-kappaB in C6 rat glioma cells after transfection with glia maturation factor. J Neurochem 74(2):596–602
Zaheer A, Lim R (1998) Overexpression of glia maturation factor (GMF) in PC12 pheochromocytoma cells activates p38 MAP kinase, MAPKAP kinase-2, and tyrosine hydroxylase. Biochem Biophys Res Commun 250(2):278–282. https://doi.org/10.1006/bbrc.1998.9301
Sanchez-Perez AM, Claramonte-Clausell B, Sanchez-Andres JV, Herrero MT (2012) Parkinson’s disease and autophagy. Parkinsons Dis 2012:429524–429526. https://doi.org/10.1155/2012/429524
Chandramani Shivalingappa P, Jin H, Anantharam V, Kanthasamy A, Kanthasamy A (2012) N-Acetyl cysteine protects against methamphetamine-induced dopaminergic neurodegeneration via modulation of redox status and autophagy in dopaminergic cells. Parkinsons Dis 2012:424285–424211. https://doi.org/10.1155/2012/424285
Wang B, Abraham N, Gao G, Yang Q (2016) Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Translational Neurodegeneration 5(1):19. https://doi.org/10.1186/s40035-016-0065-1
Zhang Z, Miah M, Culbreth M, Aschner M (2016) Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem Res 41(1–2):409–422. https://doi.org/10.1007/s11064-016-1844-x
Alers S, Loffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32(1):2–11. https://doi.org/10.1128/MCB.06159-11
Feng D, Liu L, Zhu Y, Chen Q (2013) Molecular signaling toward mitophagy and its physiological significance. Exp Cell Res 319(12):1697–1705. https://doi.org/10.1016/j.yexcr.2013.03.034
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36(6):320–328. https://doi.org/10.1016/j.tibs.2011.03.006
Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP (2011) ERK1/2 phosphorylate raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J Biol Chem 286(1):567–577. https://doi.org/10.1074/jbc.M110.159046
Memmott RM, Dennis PA (2009) Akt-dependent and independent mechanisms of mTOR regulation in cancer. Cell Signal 21(5):656–664. https://doi.org/10.1016/j.cellsig.2009.01.004
Deretic V (2012) Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol 24(1):21–31. https://doi.org/10.1016/j.coi.2011.10.006
Huang Q, Liu X, Cao C, Lei J, Han D, Chen G, Yu J, Chen L et al (2016) Apelin-13 induces autophagy in hepatoma HepG2 cells through ERK1/2 signaling pathway-dependent upregulation of Beclin1. Oncol Lett 11(2):1051–1056. https://doi.org/10.3892/ol.2015.3991
Zaheer A, Zaheer S, Sahu SK, Yang B, Lim R (2007) Reduced severity of experimental autoimmune encephalomyelitis in GMF-deficient mice. Neurochem Res 32(1):39–47. https://doi.org/10.1007/s11064-006-9220-x
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571–580. https://doi.org/10.1038/cdd.2010.191
Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P (2006) NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem 281(41):30373–30382. https://doi.org/10.1074/jbc.M602097200
Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C (2009) p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol 29(10):2594–2608. https://doi.org/10.1128/MCB.01396-08
Dan HC, Baldwin AS (2008) Differential involvement of IkappaB kinases alpha and beta in cytokine- and insulin-induced mammalian target of rapamycin activation determined by Akt. J Immunol 180(11):7582–7589
Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, Rosner M, Zlabinger GJ et al (2011) Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood 117(16):4273–4283. https://doi.org/10.1182/blood-2010-09-310888
Weichhart T, Hengstschlager M, Linke M (2015) Regulation of innate immune cell function by mTOR. Nat Rev Immunol 15(10):599–614. https://doi.org/10.1038/nri3901
Ding WX, Yin XM (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393(7):547–564. https://doi.org/10.1515/hsz-2012-0119
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. https://doi.org/10.1016/j.cell.2011.10.026
Trocoli A, Djavaheri-Mergny M (2011) The complex interplay between autophagy and NF-κB signaling pathways in cancer cells. Am J Cancer Res 1(5):629–649
Hu ZY, Chen B, Zhang JP, Ma YY (2017) Upregulation of autophagy-related gene 5 protects dopaminergic neurons in a zebrafish model of Parkinson’s disease. J Biol Chem 292:18062–18074. https://doi.org/10.1074/jbc.M116.764795
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–889. https://doi.org/10.1038/nature04724
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. https://doi.org/10.1038/nature04723
Hu Z, Zhang J, Zhang Q (2011) Expression pattern and functions of autophagy-related gene atg5 in zebrafish organogenesis. Autophagy 7(12):1514–1527
Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH (2009) Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis 35(3):385–398. https://doi.org/10.1016/j.nbd.2009.05.023
Remondelli P, Renna M (2017) The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front Mol Neurosci 10:187. https://doi.org/10.3389/fnmol.2017.00187
Kim DS, Kim JH, Lee GH, Kim HT, Lim JM, Chae SW, Chae HJ, Kim HR (2010) p38 mitogen-activated protein kinase is involved in endoplasmic reticulum stress-induced cell death and autophagy in human gingival fibroblasts. Biol Pharm Bull 33(4):545–549
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403(6765):98–103. https://doi.org/10.1038/47513
Mishra R, Karande AA (2014) Endoplasmic reticulum stress-mediated activation of p38 MAPK, caspase-2 and caspase-8 leads to abrin-induced apoptosis. PLoS One 9(3):e92586. https://doi.org/10.1371/journal.pone.0092586
Jiang P, Gan M, Ebrahim AS, Lin WL, Melrose HL, Yen SH (2010) ER stress response plays an important role in aggregation of alpha-synuclein. Mol Neurodegener 5:56. https://doi.org/10.1186/1750-1326-5-56
Acknowledgements
This work was supported by Veterans Affairs Merit Award I01BX002477 and National Institutes of Health Grants AG048205 and NS073670 to AZ. We would like to thank Dr. Alexander Jurkevich, Associate Director of Molecular Cytology core, University of Missouri, Columbia-MO for his help in preparation and validation of confocal images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Selvakumar, G.P., Iyer, S.S., Kempuraj, D. et al. Molecular Association of Glia Maturation Factor with the Autophagic Machinery in Rat Dopaminergic Neurons: a Role for Endoplasmic Reticulum Stress and MAPK Activation. Mol Neurobiol 56, 3865–3881 (2019). https://doi.org/10.1007/s12035-018-1340-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1340-1