Abstract
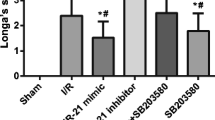
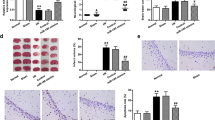
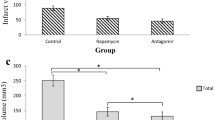
Ischemic stroke resulting from blockade of brain vessels lacks effective treatments, prompting exploration for potential therapies. Among promising candidates, microRNA-149 (miR-149) has been investigated for its role in alleviating oxidative stress, inflammation, and neurodegeneration associated with ischemic conditions. To evaluate its therapeutic effect, male Wistar rats were categorized into five groups, each consisting of 27 rats: sham, MCAO, lentiviral control, lentiviral miR-149, and miR149-5p mimic. Treatments were microinjected intracerebroventricularly (ICV) (right side), and ischemia was induced using middle cerebral artery occlusion (MCAO) procedure. Post-MCAO, neurological function, histopathological changes, blood-brain barrier (BBB) permeability, cerebral edema, and mRNA levels of Fas ligand (Faslg) and glutamate ionotropic NMDA receptor 1 (GRIN1) were assessed, alongside biochemical assays. MiR-149 administration improved neurological function, reduced brain damage, preserved BBB integrity, and attenuated cerebral edema. Upregulation of miR149-5p decreased Faslg and GRIN1 expression in ischemic brain regions. MiR-149 also reduced oxidative stress, enhanced antioxidant activity, decreased caspase-1 and − 3 activity, and modulated inflammatory factors in ischemic brain regions. Moreover, DNA fragmentation as an index of cell death decreased following miR-149 treatment. In conclusion, the study underscores miR-149 potential as a neuroprotective agent against ischemic stroke, showcasing its efficacy in modulating various mechanisms and supporting its candidacy as a promising therapeutic target for innovative strategies in stroke treatment.












Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Kuriakose D, Xiao Z (Oct. 2020) Pathophysiology and treatment of stroke: Present Status and Future perspectives. Int J Mol Sci 21(20):7609. https://doi.org/10.3390/ijms21207609
An SJ, Kim TJ, Yoon B-W (Jan. 2017) Epidemiology, risk factors, and clinical features of Intracerebral Hemorrhage: an update. J Stroke 19(1):3–10. https://doi.org/10.5853/jos.2016.00864
Migdady I, Russman A, Buletko AB (2021) Atrial Fibrillation and Ischemic Stroke: A Clinical Review, Semin. Neurol, vol. 41, no. 4, pp. 348–364, Aug. https://doi.org/10.1055/s-0041-1726332
Wajngarten M, Silva GS (Jul. 2019) Hypertension and stroke: update on treatment. Eur Cardiol Rev 14(2):111–115. https://doi.org/10.15420/ecr.2019.11.1
Bulygin KV et al (2020) Sep., Can miRNAs Be Considered as Diagnostic and Therapeutic Molecules in Ischemic Stroke Pathogenesis?-Current Status, Int. J. Mol. Sci, vol. 21, no. 18, p. 6728, https://doi.org/10.3390/ijms21186728
Liang Y et al (2018) Inhibition of MiRNA-125b decreases cerebral Ischemia/Reperfusion Injury by Targeting CK2α/NADPH oxidase signaling. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 45(5):1818–1826. https://doi.org/10.1159/000487873
Aldous EK et al (2022) Mar., Identification of Novel Circulating miRNAs in Patients with Acute Ischemic Stroke, Int. J. Mol. Sci, vol. 23, no. 6, p. 3387, https://doi.org/10.3390/ijms23063387
Ren F, Yao Y, Cai X, Cai Y, Su Q, Fang G (2021) MiR-149-5p: An Important miRNA Regulated by Competing Endogenous RNAs in Diverse Human Cancers, Front. Oncol, vol. 11, p. 743077, Oct. https://doi.org/10.3389/fonc.2021.743077
Ruan D, Liu Y, Wang X, Yang D, Sun Y (2019) miR-149-5p protects against high glucose-induced pancreatic beta cell apoptosis via targeting the BH3-only protein BIM, Exp. Mol. Pathol, vol. 110, p. 104279, Oct. https://doi.org/10.1016/j.yexmp.2019.104279
Khidr EG et al (Aug. 2023) The potential role of miRNAs in the pathogenesis of cardiovascular diseases - a focus on signaling pathways interplay. Pathol Res Pract 248:154624. https://doi.org/10.1016/j.prp.2023.154624
Ghasemloo E, Oryan S, Bigdeli MR, Mostafavi H, Eskandari M (2021) The neuroprotective effect of MicroRNA-149-5p and coenzymeQ10 by reducing levels of inflammatory cytokines and metalloproteinases following focal brain ischemia in rats, Brain Res. Bull, vol. 169, pp. 205–213, Apr. https://doi.org/10.1016/j.brainresbull.2021.01.013
Wan Y et al (2018) Jun., MicroRNA-149-5p regulates blood-brain barrier permeability after transient middle cerebral artery occlusion in rats by targeting S1PR2 of pericytes, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol, vol. 32, no. 6, pp. 3133–3148, https://doi.org/10.1096/fj.201701121R
Qin C, Lv Y, Zhao H, Yang B, Zhang P (2019) MicroRNA-149 Suppresses Inflammation in Nucleus Pulposus Cells of Intervertebral Discs by Regulating MyD88, Med. Sci. Monit. Int. Med. J. Exp. Clin. Res, vol. 25, pp. 4892–4900, Jul. https://doi.org/10.12659/MSM.915858
Tiscornia G, Singer O, Verma IM (2006) Production and purification of lentiviral vectors. Nat Protoc 1(1):241–245. https://doi.org/10.1038/nprot.2006.37
Longa EZ, Weinstein PR, Carlson S, Cummins R (Jan. 1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91. https://doi.org/10.1161/01.str.20.1.84
Chen J et al (Apr. 2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011. https://doi.org/10.1161/01.str.32.4.1005
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume, J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab, vol. 10, no. 2, pp. 290–293, Mar. https://doi.org/10.1038/jcbfm.1990.47
Torfeh A, Abdolmaleki Z, Nazarian S, Shirazi Beheshtiha SH (2021) Modafinil-coated nanoparticle increases expressions of brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor and neuronal nuclear protein, and protects against middle cerebral artery occlusion-induced neuron apoptosis in the rat hippocampus, Anat. Rec. Hoboken NJ 2007, vol. 304, no. 9, pp. 2032–2043, Sep. https://doi.org/10.1002/ar.24581
Asgari Taei A, Dargahi L, Khodabakhsh P, Kadivar M, Farahmandfar M (2022) Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke, CNS Neurosci. Ther, vol. 28, no. 9, pp. 1425–1438, Sep. https://doi.org/10.1111/cns.13886
Fernández-López D et al (Jul. 2012) Blood–brain barrier permeability is increased after Acute Adult Stroke but not neonatal stroke in the rat. J Neurosci 32:9588–9600. https://doi.org/10.1523/JNEUROSCI.5977-11.2012
Yang C et al (Dec. 2021) Neurovascular protection by adropin in experimental ischemic stroke through an endothelial nitric oxide synthase-dependent mechanism. Redox Biol 48:102197. https://doi.org/10.1016/j.redox.2021.102197
Alizadeh Makvandi A, Khalili M, Roghani M, Amiri Moghaddam S (2021) Hesperetin ameliorates electroconvulsive therapy-induced memory impairment through regulation of hippocampal BDNF and oxidative stress in a rat model of depression, J. Chem. Neuroanat, vol. 117, p. 102001, Nov. https://doi.org/10.1016/j.jchemneu.2021.102001
Lee JW, Lee D-H, Park JK, Han JS (2019) Sodium nitrite-derived nitric oxide protects rat testes against ischemia/reperfusion injury. Asian J Androl 21(1):92–97. https://doi.org/10.4103/aja.aja_76_18
Silva-Islas CA, Chánez-Cárdenas ME, Barrera-Oviedo D, Ortiz-Plata A, Pedraza-Chaverri J, Maldonado PD (2019) Diallyl Trisulfide Protects Rat Brain Tissue against the Damage Induced by Ischemia-Reperfusion through the Nrf2 Pathway, Antioxidants, vol. 8, no. 9, p. 410, Sep. https://doi.org/10.3390/antiox8090410
De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD (2012) Extracellular chromatin is an important mediator of ischemic stroke in mice, Arterioscler. Thromb. Vasc. Biol, vol. 32, no. 8, pp. 1884–1891, Aug. https://doi.org/10.1161/ATVBAHA.112.250993
Khaleghi-Mehr M, Delshad A-A, Shafie-Damavandi S, Roghani M (2023) Metformin mitigates amyloid β1-40-induced cognitive decline via attenuation of oxidative/nitrosative stress and neuroinflammation, Metab. Brain Dis, vol. 38, no. 4, pp. 1127–1142, Apr. https://doi.org/10.1007/s11011-023-01170-1
Albazal A, Delshad A-A, Roghani M (Mar. 2021) Melatonin reverses cognitive deficits in streptozotocin-induced type 1 diabetes in the rat through attenuation of oxidative stress and inflammation. J Chem Neuroanat 112:101902. https://doi.org/10.1016/j.jchemneu.2020.101902
Balbinot G, Schuch CP (2019) Compensatory Relearning Following Stroke: Cellular and Plasticity Mechanisms in Rodents, Front. Neurosci, vol. 12, Accessed: Nov. 24, 2023. [Online]. Available: https://www.frontiersin.org/articles/https://doi.org/10.3389/fnins.2018.01023
Lima RR et al (2016) Neurodegeneration and glial response after Acute Striatal Stroke: histological basis for neuroprotective studies. Oxid Med Cell Longev 2016:3173564. https://doi.org/10.1155/2016/3173564
Nian K, Harding IC, Herman IM, Ebong EE (2020) Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction, Front. Physiol, vol. 11, Accessed: Dec. 01, 2023. [Online]. Available: https://www.frontiersin.org/articles/https://doi.org/10.3389/fphys.2020.605398
Sun P, Hamblin MH, Yin K-J (Mar. 2022) Non-coding RNAs in the regulation of blood–brain barrier functions in central nervous system disorders. Fluids Barriers CNS 19:27. https://doi.org/10.1186/s12987-022-00317-z
Kamal FZ, Lefter R, Jaber H, Balmus I-M, Ciobica A, Iordache A-C (2023) The Role of Potential Oxidative Biomarkers in the Prognosis of Acute Ischemic Stroke and the Exploration of Antioxidants as Possible Preventive and Treatment Options, Int. J. Mol. Sci, vol. 24, no. 7, p. 6389, Mar. https://doi.org/10.3390/ijms24076389
Jelinek M, Jurajda M, Duris K (2021) Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke, Antioxidants, vol. 10, no. 12, Art. no. 12, Dec. https://doi.org/10.3390/antiox10121886
Deng Z et al (2022) Sep., Leonurine Reduces Oxidative Stress and Provides Neuroprotection against Ischemic Injury via Modulating Oxidative and NO/NOS Pathway, Int. J. Mol. Sci, vol. 23, no. 17, https://doi.org/10.3390/ijms231710188
Zhang Q et al (2017) Jun., MicroRNA-149* suppresses hepatic inflammatory response through antagonizing STAT3 signaling pathway, Oncotarget, vol. 8, no. 39, pp. 65397–65406, https://doi.org/10.18632/oncotarget.18541
Jiang Y, Zhang L, Tian H (Apr. 2023) MicroRNA-149 improves osteoarthritis via repression of VCAM-1 and inactivation of PI3K/AKT pathway. Exp Gerontol 174:112103. https://doi.org/10.1016/j.exger.2023.112103
Lang H et al (Aug. 2017) MicroRNA-149 contributes to scarless wound healing by attenuating inflammatory response. Mol Med Rep 16(2):2156–2162. https://doi.org/10.3892/mmr.2017.6796
Lin J, Lin H, Ma C, Dong F, Hu Y, Li H (2019) MiR-149 Aggravates Pyroptosis in Myocardial Ischemia-Reperfusion Damage via Silencing FoxO3, Med. Sci. Monit. Int. Med. J. Exp. Clin. Res, vol. 25, pp. 8733–8743, Nov. https://doi.org/10.12659/MSM.918410
Wang C et al (2023) Jan., Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons, Molecules, vol. 28, no. 7, Art. no. 7, https://doi.org/10.3390/molecules28073181
He Y, Yu D, Zhu L, Zhong S, Zhao J, Tang J (Jan. 2018) miR-149 in Human Cancer: a systemic review. J Cancer 9(2):375–388. https://doi.org/10.7150/jca.21044
Ye X et al (2022) Caspase-1: A Promising Target for Preserving Blood–Brain Barrier Integrity in Acute Stroke, Front. Mol. Neurosci, vol. 15, Accessed: Jan. 19, 2024. [Online]. Available: https://www.frontiersin.org/articles/https://doi.org/10.3389/fnmol.2022.856372
Liang Y et al (2021) Inhibition of Caspase-1 Ameliorates Ischemia-Associated Blood-Brain Barrier Dysfunction and Integrity by Suppressing Pyroptosis Activation, Front. Cell. Neurosci, vol. 14, Accessed: Jan. 19, 2024. [Online]. Available: https://www.frontiersin.org/articles/https://doi.org/10.3389/fncel.2020.540669
Mao R, Zong N, Hu Y, Chen Y, Xu Y (2022) Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke, Neurosci. Bull, vol. 38, no. 10, pp. 1229–1247, Oct. https://doi.org/10.1007/s12264-022-00859-0
Uzdensky AB (2019) Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins, Apoptosis Int. J. Program. Cell Death, vol. 24, no. 9–10, pp. 687–702, Oct. https://doi.org/10.1007/s10495-019-01556-6
Dailah HG (2022) Potential of Therapeutic Small Molecules in Apoptosis Regulation in the Treatment of Neurodegenerative Diseases: An Updated Review, Mol. Basel Switz, vol. 27, no. 21, p. 7207, Oct. https://doi.org/10.3390/molecules27217207
Aycan A et al (Jan. 2023) Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage. Transl Neurosci 14(1):20220311. https://doi.org/10.1515/tnsci-2022-0311
Acknowledgements
No acknowledgments.
Funding
This study received support from a grant (No. 99025398) provided by the Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Contributions
The study’s conception and design were developed by M.R.B. and M.R., with H.S. and S.A. providing support in procuring necessary equipment. S.V. carried out the experiments, analyzed the data, and drafted the initial manuscript. The culmination of the manuscript was a collaborative effort, with all authors contributing equally to its refinement.
Corresponding authors
Ethics declarations
Ethics Approval
The animal experiments adhered to the National Institute of Health Guide for the Care and Use of Laboratory Animals. The study protocol received approval from the Research Ethics Committees of Shahid Beheshti University (IR.SBU.REC.1400.100), ensuring compliance with ethical standards in research involving laboratory animals.
Consent to Participate
Not Applicable.
Consent for Publication
Not Applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vahidi, S., Bigdeli, MR., Shahsavarani, H. et al. Neuroprotective Therapeutic Potential of microRNA-149-5p against Murine Ischemic Stroke. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04159-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04159-8