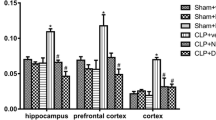
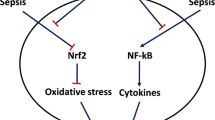
Abstract
Neurological dysfunction as a result of neuroinflammation has been reported in sepsis and cause high mortality. High levels of cytokines stimulate the formation of neurotoxic metabolites by kynurenine (KYN) pathway. Vitamin B6 (vit B6) has anti-inflammatory and antioxidant properties and also acts as a cofactor for enzymes of the KYN pathway. Thus, by using a relevant animal model of polymicrobial sepsis, we studied the effect of vit B6 on the KYN pathway, acute neurochemical and neuroinflammatory parameters, and cognitive dysfunction in rats. Male Wistar rats (250–300 g) were submitted to cecal ligation and perforation (CLP) and divided into sham + saline, sham + vit B6, CLP + saline, and CLP + vit B6 (600 mg/kg, s.c.) groups. Twenty-four hours later, the prefrontal cortex and hippocampus were removed for neurochemical and neuroinflammatory analyses. Animals were followed for 10 days to determine survival rate, when cognitive function was assessed by behavioral tests. Vitamin B6 interfered in the activation of kynurenine pathway, which led to an improvement in neurochemical and neuroinflammatory parameters and, consequently, in the cognitive functions of septic animals. Thus, the results indicate that vit B6 exerts neuroprotective effects in acute and late consequences after sepsis.









Similar content being viewed by others
References
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150. doi:10.1056/NEJMra021333
Ely EW (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA J Am Med Assoc 291:1753–1762. doi:10.1001/jama.291.14.1753
Lazosky A, Young GB, Ba SZ, Phillips R (2010) Quality of life after septic illness. J Crit Care 25:406–412. doi:10.1016/j.jcrc.2009.10.001
Zhang L, Wang X, Ai Y et al (2012) Epidemiological features and risk factors of sepsis-associated encephalopathy in intensive care unit patients: 2008–2011. Chin Med J 125:2008–2011. doi:10.3760/cma.j.issn.0366-6999.2012.05.018
Silvestre F, Danielski LG, Michels M et al (2014) Effects of organoselenium compounds on early and late brain biochemical alterations in sepsis-survivor rats. Neurotox Res 26:382–391. doi:10.1007/s12640-014-9475-y
Adam N, Kandelman S, Mantz J, et al (2013) Sepsis-induced brain dysfunction. Expert Rev Anti Infect Ther 11(2):211-221. doi:10.1586/eri.12.159.
Taccone FS, Su F, De Deyne C et al (2013) Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med 42:1–9. doi:10.1097/CCM.0b013e3182a641b8
Lemstra AW, Groen in’t Woud JC, Hoozemans JJ, et al (2007) Microglia activation in sepsis: a case-control study. J Neuroinflammation 4:4. doi:10.1186/1742-2094-4-4
Comim CM, Rezin GT, Scaini G et al (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318. doi:10.1016/j.mito.2008.07.002
Rogers AJ, McGeachie M, Baron RM et al (2014) Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS One 9:e87538. doi:10.1371/journal.pone.0087538
Tattevin P, Monnier D, Tribut O et al (2010) Enhanced Indoleamine 2, 3-dioxygenase activity in patients with severe sepsis and septic shock. J Infect Dis 201(6):956–966. doi:10.1086/650996
Maddison DC, Giorgini F (2015) The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol. doi:10.1016/j.semcdb.2015.03.002
Campbell BM, Charych E, Lee AW, Möller T (2014) Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 8:12. doi:10.3389/fnins.2014.00012
Midttun O, Ulvik A, Ringdal Pedersen E et al (2011) Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr 141:611–617. doi:10.3945/jn.110.133082
Huang Y-C, Lan P-H, Cheng C-H et al (2002) Vitamin B6 intakes and status of mechanically ventilated critically ill patients in Taiwan. Eur J Clin Nutr 56:387–392. doi:10.1038/sj.ejcn.1601321
Huang Y-C, Chang H-H, Huang S-C et al (2005) Plasma pyridoxal 5′-phosphate is a significant indicator of immune responses in the mechanically ventilated critically ill. Nutrition 21:779–785. doi:10.1016/j.nut.2004.11.013
Hou C-T, Wu Y-H, Huang P-N et al (2011) Higher plasma pyridoxal 5′-phosphate is associated with better blood glucose responses in critically ill surgical patients with inadequate vitamin B-6 status. Clin Nutr 30:478–483. doi:10.1016/j.clnu.2011.01.014
Cheng CH, Huang SC, Chiang TY et al (2013) Higher plasma pyridoxal phosphate is associated with increased antioxidant enzyme activities in critically ill surgical patients. Biomed Res Int 2013:572081. doi:10.1155/2013/572081
Cheng CH, Chang SJ, Lee BJ et al (2006) Vitamin B 6 supplementation increases immune responses in critically ill patients. Eur J Clin Nutr 60(10):1207–1213. doi:10.1038/sj.ejcn.1602439
Fink MP, Heard SO (1990) Laboratory models of sepsis and septic shock. J Surg Res 49:186–196
Barichello T, Generoso JS, Simoes LR et al (2014) Vitamin B6 prevents cognitive impairment in experimental pneumococcal meningitis. Exp Biol Med (Maywood) 239(10):1360–1365
Paxinos G, Watson C (2013) The Rat brain in stereotaxic coordinates, 7th edn. Elsevier, Cambridge
Kwidzinski E, Bunse J, Aktas O et al (2005) Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J 19:1347–1349. doi:10.1096/fj.04-3228fje
Uyama O, Okamura N, Yanase M et al (1988) Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab 8:282–284. doi:10.1038/jcbfm.1988.59
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–341
Green LC, Wagner DA, Glogowski J, et al (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1):131–138.
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421-431.
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346-357.
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316. doi:10.1006/abbi.1996.0178
Fischer J, Ruitenbeek W, Ja B et al (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Rustin P, Chretien D, Bourgeron T et al (1994) Biochemical and molecular investigations in respiratory chain. Clin Chim Acta 228:35–51
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
de Lima MNM, Laranja DC, Caldana F et al (2005) Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol 40:506–511. doi:10.1016/j.exger.2005.03.004
Tuon L, Comim CM, Petronilho F et al (2008) Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med 34:1724–1731. doi:10.1007/s00134-008-1129-1
Barichello T, Martins MR, Reinke A et al (2005) Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33:221–223. doi:10.1097/01.CCM.0000150741.12906.BD
Vianna MR, Izquierdo LA, Barros DM et al (2000) Short- and long-term memory: differential involvement of neurotransmitter systems and signal transduction cascades. An Acad Bras Cienc 72:353–364
Okada K, Angkawidjaja C, Koga Y, Kanaya S (2014) Structural and mechanistic insights into the kynurenine aminotransferase-mediated excretion of kynurenic acid. J Struct Biol 185:257–266. doi:10.1016/j.jsb.2014.01.009
Reyes Ocampo J, Lugo Huitrón R, González-esquivel D et al (2014) Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxidative Med Cell Longev 2014:646909. doi:10.1155/2014/646909
Semmler A, Okulla T, Sastre M et al (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 30:144–157. doi:10.1016/j.jchemneu.2005.07.003
Sonneville R, Verdonk F, Rauturier C et al (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3:1–11. doi:10.1186/2110-5820-3-15
Kanai M, Funakoshi H, Takahashi H et al (2009) Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain 2:8. doi:10.1186/1756-6606-2-8
Murakami Y, Saito K (2013) Species and cell types difference in tryptophan metabolism. Int J Tryptophan Res 6:47–54. doi:10.4137/IJTR.S11558
Changsirivathanathamrong D, Wang Y, Rajbhandari D et al (2011) Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit Care Med 39:1. doi:10.1097/CCM.0b013e31822827f2
Corona AW, Norden DM, Skendelas JP et al (2013) Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX3CR1)-deficient mice. Brain Behav Immun 31:134–142. doi:10.1016/j.bbi.2012.08.008
Lawson MA, Parrott JM, McCusker RH et al (2013) Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J Neuroinflammation 10:87. doi:10.1186/1742-2094-10-87
Wang Y, Lawson MA, Kelley KW, Dantzer R (2011) Primary murine microglia are resistant to nitric oxide inhibition of indoleamine 2, 3 dioxygenase. Brain Behav Immun 24:1249–1253. doi:10.1016/j.bbi.2010.04.015.Primary
Michels M, Danielski LG, Vieira A et al (2015) CD40–CD40 ligand pathway is a major component of acute neuroinflammation and contributes to long-term cognitive dysfunction after sepsis. Mol Med 21:219–226. doi:10.2119/molmed.2015.00070
Quan N, Banks W a. (2007) Brain-immune communication pathways. Brain Behav Immun 21:727–735. doi:10.1016/j.bbi.2007.05.005
Dal-Pizzol F, Rojas HA, Dos Santos EM et al (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 48:62–70. doi:10.1007/s12035-013-8433-7
Comim CM, Vilela MC, Constantino LS et al (2011) Traffic of leukocytes and cytokine up-regulation in the central nervous system in sepsis. Intensive Care Med 37:711–718. doi:10.1007/s00134-011-2151-2
Kacimi R, Giffard RG, Yenari MA (2011) Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J Inflamm (Lond) 8:7. doi:10.1186/1476-9255-8-7
Yanaka N, Koyama T-A, Komatsu S-I et al (2005) Vitamin B6 suppresses NF-kappaB activation in LPS-stimulated mouse macrophages. Int J Mol Med 16:1071–1075
Zhang P, Tsuchiya K, Kinoshita T et al (2016) Vitamin B6 prevents IL-1β production by inhibiting NLRP3 inflammasome activation. J Biol Chem. doi:10.1074/jbc.M116.743815
Stamler JS (1994) Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78:931–936. doi:10.1016/0092-8674(94)90269-0
Lobo SM, Soriano FG, Barbeiro DF et al (2009) Effects of dobutamine on gut mucosal nitric oxide production during endotoxic shock in rabbits. Med Sci Monit 15:BR37–BR42
Berg RMG, Møller K, Bailey DM (2011) Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab 31:1532–1544. doi:10.1038/jcbfm.2011.48
Santiago APSA, Chaves EA, Oliveira MF, Galina A (2008) Reactive oxygen species generation is modulated by mitochondrial kinases: correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie 90:1566–1577. doi:10.1016/j.biochi.2008.06.013
Duma D, Fernandes D, Bonini MG et al (2011) NOS-1-derived NO is an essential triggering signal for the development of systemic inflammatory responses. Eur J Pharmacol 668:285–292. doi:10.1016/j.ejphar.2011.05.065
Fortin CF, McDonald PP, Fülöp T, Lesur O (2010) Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock 33:344–352. doi:10.1097/SHK.0b013e3181c0f068
Coleman N (2001) Antioxidants in critical care medicine. Environ Toxicol Pharmacol 10:183–188
Kanouchi H (2013) Low pyridoxine concentrations enhance lipopolysaccharide-stimulated gene expression of cyclooxygenase-2 and inducible nitric oxide synthase in RAW264.7 cells. J Nutr Sci Vitaminol 7:548–551
Matxain JM, Ristila M, Eriksson LA (2006) Theoretical study of the antioxidant properties of pyridoxine. J Phys Chem A. 110(48):13068–13072
Kannan K, Jain SK (2004) Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2o2-treated U937 monocytes. Free Radic Biol Med 36:423–428. doi:10.1016/j.freeradbiomed.2003.09.012
Choi J, Koh S (2008) Role of brain inflammation in epileptogenesis. Yonsei Med J 49(1):1–18. doi:10.3349/ymj.2008.49.1.1
Rommer PS, Leblhuber F (2016) Lowered levels of carbonyl proteins after vitamin B supplementation in patients with mild cognitive impairment and Alzheimer’s disease. Neurodegener Dis 16(3-4):284–289. doi:10.1159/000441565
Stone TW, Darlington LG (2013) The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol 169:1211–1227. doi:10.1111/bph.12230
Lugo-huitrón R, Blanco-ayala T, Ugalde-muñiz P et al (2011) Neurotoxicology and teratology on the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol 33(5):538–547. doi:10.1016/j.ntt.2011.07.002
Bilski P, Li MY, Ehrenshaft M et al (2000) Symposium-in-print Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol 71:129–134
Taş S, Sarandöl E, Dirican M (2014) Vitamin B6 supplementation improves oxidative stress and enhances serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. ScientificWorldJournal. doi:10.1155/2014/351598
Kowaltowski AJ, de Souza-pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343. doi:10.1016/j.freeradbiomed.2009.05.004
Mooney S, Leuendorf J, Hendrickson C, Hellmann H (2009) Vitamin B6: a long known compound of surprising complexity. Molecules 14(1):329–351. doi:10.3390/molecules14010329
Gao R, Kan MQ, Wang SG et al (2015) Disrupted tryptophan metabolism induced cognitive impairment in a mouse model of sepsis-associated encephalopathy. Inflammation. doi:10.1007/s10753-015-0279-x
Götz ME, Künig G, Riederer P, Youdim MB (1994) Oxidative stress: free radical production in neural degeneration. Pharmacol Ther 63:37–122.
Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. J Am Med Assoc 304:1787–1794. doi:10.1001/jama.2010.1553
Kaur J, Singhi P, Singhi S et al (2015) Neurodevelopmental and behavioral outcomes in children with sepsis-associated encephalopathy admitted to pediatric intensive care unit: a prospective case control study. J Child Neurol. doi:10.1177/0883073815610431
Chess AC, Simoni MK, Alling TE, Bucci DJ (2007) Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull 33:797–804. doi:10.1093/schbul/sbl033
Heisler JM, Connor JCO (2015) Brain, behavior, and immunity metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun 2014:1–10. doi:10.1016/j.bbi.2015.06.022
Parrot J, Redus L, O’Connor JC (2016) Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation 13:124
Guo C, Wang P, Zhong M-L et al (2013) Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int 62:165–172. doi:10.1016/j.neuint.2012.12.005
Finglas PM (2000) Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Trends Food Sci Technol. doi:10.1016/S0924-2244(01)00010-3
Shabbir F, Patel A, Mattison C et al (2013) Neurochemistry international effect of diet on serotonergic neurotransmission in depression. Neurochem Int 62:324–329. doi:10.1016/j.neuint.2012.12.014
Acknowledgements
This research was supported by grants from CNPq (FP). FP, TB, FD-P, and RSC are CNPq Research Fellows.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors or funding sources has conflict of interest.
Rights and permissions
About this article
Cite this article
Danielski, L.G., Giustina, A.D., Goldim, M.P. et al. Vitamin B6 Reduces Neurochemical and Long-Term Cognitive Alterations After Polymicrobial Sepsis: Involvement of the Kynurenine Pathway Modulation. Mol Neurobiol 55, 5255–5268 (2018). https://doi.org/10.1007/s12035-017-0706-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0706-0