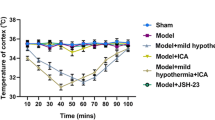
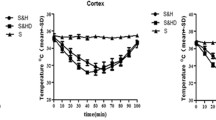
Abstract
Therapeutic hypothermia (TH) is the most potent therapeutic strategy for global cerebral ischemia (GCI), usually induced by cardiac arrest. TH has been shown both to suppress the delayed neuronal cell death in the vulnerable hippocampal CA1 subregion and to improve neurological outcomes in experimental animals after GCI. However, given the multiple adverse effects resulting from TH, application of such a therapy is typically limited. In recent years, methylene blue (MB) has emerged as a potential therapeutic drug for the treatment of neurodegenerative diseases. In this study, we investigated the beneficial effects of mild TH combined with MB treatment after GCI. We report that both the neuronal survival in the hippocampal CA1 region and the hippocampus-dependent spatial learning and memory in the combined treatment animals were enhanced compared to those in the single treatment animals. Mechanistic studies revealed that combined TH and MB treatment significantly attenuated mitochondrial dysfunction induced by GCI in the hippocampus CA1 region. The combined treatment also markedly suppressed GCI-induced reactive gliosis and inflammation and reduced oxidative stress while enhancing the antioxidant capacity of hippocampal CA1 neurons. Finally, combining TH and MB synergistically attenuated the intrinsic cytochrome c/caspase-3 apoptotic pathway induced by GCI. Our results suggest that TH and MB act synergistically to protect the ischemic brain and suppress cognitive impairment caused by GCI.








Similar content being viewed by others
References
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(6):724–738. doi:10.1016/j.cmet.2011.08.016
Kennedy C, Sokoloff L (1957) An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest 36(7):1130–1137. doi:10.1172/JCI103509
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36(10):587–597. doi:10.1016/j.tins.2013.07.001
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346(8):557–563. doi:10.1056/NEJMoa003289
Wolman RL, Nussmeier NA, Aggarwal A, Kanchuger MS, Roach GW, Newman MF, Mangano CM, Marschall KE et al (1999) Cerebral injury after cardiac surgery: identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke; a journal of cerebral circulation 30(3):514–522
Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K et al (1996) Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med 335(25):1857–1863. doi:10.1056/NEJM199612193352501
Harukuni I, Bhardwaj A (2006) Mechanisms of brain injury after global cerebral ischemia. Neurol Clin 24(1):1–21. doi:10.1016/j.ncl.2005.10.004
Weitzdoerfer R, Pollak A, Lubec B (2004) Perinatal asphyxia in the rat has lifelong effects on morphology, cognitive functions, and behavior. Semin Perinatol 28(4):249–256
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79(4):1431–1568
Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995) Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke; a journal of cerebral circulation 26(8):1478–1489
Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW (2012) Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms?: a systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation 83(2):188–196. doi:10.1016/j.resuscitation.2011.07.031
Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bbttiger BW, Callaway C, Clark RS et al (2010) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II). International emergency nursing 18(1):8–28. doi:10.1016/j.ienj.2009.07.001
Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J (2011a) Hypothermia after cardiac arrest should be further evaluated—a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol 151(3):333–341. doi:10.1016/j.ijcard.2010.06.008
Perbet S, Mongardon N, Dumas F, Bruel C, Lemiale V, Mourvillier B, Carli P, Varenne O et al (2011) Early-onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med 184(9):1048–1054. doi:10.1164/rccm.201102-0331OC
Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P, Nilsson F, Friberg H (2011b) Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med 39(1):57–64. doi:10.1097/CCM.0b013e3181fa4301
Sunde K (2013) Therapeutic hypothermia in cardiac arrest. Revista espanola de cardiologia 66(5):346–349. doi:10.1016/j.rec.2012.10.004
Jiang Z, Duong TQ (2016) Methylene blue treatment in experimental ischemic stroke: a mini review. Brain circulation 2(1):48–53. doi:10.4103/2394-8108.178548
Schirmer RH, Adler H, Pickhardt M, Mandelkow E (2011) Lest we forget you—methylene blue. Neurobiol Aging 32(12):2325 e2327–2325 e2316. doi:10.1016/j.neurobiolaging.2010.12.012
Oz M, Lorke DE, Petroianu GA (2009) Methylene blue and Alzheimer’s disease. Biochem Pharmacol 78(8):927–932. doi:10.1016/j.bcp.2009.04.034
Ahmed ME, Tucker D, Dong Y, Lu Y, Zhao N, Wang R, Zhang Q (2016) Methylene blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience 336:39–48. doi:10.1016/j.neuroscience.2016.08.036
Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q (2016) Neuroprotective and functional improvement effects of methylene blue in global cerebral ischemia. Mol Neurobiol 53(8):5344–5355. doi:10.1007/s12035-015-9455-0
Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R et al (2011) Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem 286(18):16504–16515. doi:10.1074/jbc.M110.208447
Fang Q, Yan X, Li S, Sun Y, Xu L, Shi Z, Wu M, Lu Y et al (2016) Methylene blue ameliorates ischemia/reperfusion-induced cerebral edema: an MRI and transmission electron microscope study. Acta Neurochir Suppl 121:227–236. doi:10.1007/978-3-319-18497-5_41
Rodriguez P, Zhao J, Milman B, Tiwari YV, Duong TQ (2016) Methylene blue and normobaric hyperoxia combination therapy in experimental ischemic stroke. Brain and behavior 6(7):e00478. doi:10.1002/brb3.478
Dormoi J, Briolant S, Desgrouas C, Pradines B (2013) Impact of methylene blue and atorvastatin combination therapy on the apparition of cerebral malaria in a murine model. Malar J 12:127. doi:10.1186/1475-2875-12-127
Zoungrana A, Coulibaly B, Sie A, Walter-Sack I, Mockenhaupt FP, Kouyate B, Schirmer RH, Klose C et al (2008) Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. PLoS One 3(2):e1630. doi:10.1371/journal.pone.0001630
Xu CH, Yu LK, Cao L, Yang R, Yan J, Liu ZC, Wang Y (2016) Value of radial probe endobronchial ultrasound-guided localization of solitary pulmonary nodules with the combination of ultrathin bronchoscopy and methylene blue prior to video-assisted thoracoscopic surgery. Molecular and clinical oncology 5(2):279–282. doi:10.3892/mco.2016.913
Coulibaly B, Zoungrana A, Mockenhaupt FP, Schirmer RH, Klose C, Mansmann U, Meissner PE, Muller O (2009) Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One 4(5):e5318. doi:10.1371/journal.pone.0005318
D'Amico F (2005) A polychromatic staining method for epoxy embedded tissue: a new combination of methylene blue and basic fuchsine for light microscopy. Biotechnic & histochemistry : official publication of the Biological Stain Commission 80(5–6):207–210. doi:10.1080/10520290600560897
Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, Yang F, Reddy Sareddy G et al (2013) Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain : a journal of neurology 136(Pt 5):1432–1445. doi:10.1093/brain/awt046
Oh JS, Kim SW, Cho HJ, Kyong YY, Oh YM, Choi SM, Choi KH, Park KN (2013) Combination treatment with 17beta-estradiol and therapeutic hypothermia for transient global cerebral ischemia in rats. Am J Emerg Med 31(1):154–160. doi:10.1016/j.ajem.2012.06.033
Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW (2009) Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. The Journal of neuroscience : the official journal of the Society for Neuroscience 29(44):13823–13836. doi:10.1523/JNEUROSCI.3574-09.2009
Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC et al (2017) Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging 49:165–182. doi:10.1016/j.neurobiolaging.2016.10.003
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clinica chimica acta; international journal of clinical chemistry 329(1–2):23–38
Andresen M, Gazmuri JT, Marin A, Regueira T, Rovegno M (2015) Therapeutic hypothermia for acute brain injuries. Scandinavian journal of trauma, resuscitation and emergency medicine 23:42. doi:10.1186/s13049-015-0121-3
Zhang H, Zhou M, Zhang J, Mei Y, Sun S, Tong E (2008) Therapeutic effect of post-ischemic hypothermia duration on cerebral ischemic injury. Neurol Res 30(4):332–336. doi:10.1179/174313208X300279
Racay P, Tatarkova Z, Chomova M, Hatok J, Kaplan P, Dobrota D (2009) Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem Res 34(8):1469–1478. doi:10.1007/s11064-009-9934-7
Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Perez-Pinzon MA (2001) Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 21(12):1401–1410. doi:10.1097/00004647-200112000-00004
Li PA, Kristian T, He QP, Siesjo BK (2000) Cyclosporin A enhances survival, ameliorates brain damage, and prevents secondary mitochondrial dysfunction after a 30-minute period of transient cerebral ischemia. Exp Neurol 165(1):153–163. doi:10.1006/exnr.2000.7459
Cafe C, Torri C, Gatti S, Adinolfi D, Gaetani P, Rodriguez YBR, Marzatico F (1994) Changes in non-synaptosomal and synaptosomal mitochondrial membrane-linked enzymatic activities after transient cerebral ischemia. Neurochem Res 19(12):1551–1555
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348(26):2656–2668. doi:10.1056/NEJMra022567
Sims NR (1992) Energy metabolism and selective neuronal vulnerability following global cerebral ischemia. Neurochem Res 17(9):923–931
Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J et al (2013) Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 369(23):2197–2206. doi:10.1056/NEJMoa1310519
Yang SH, Li W, Sumien N, Forster M, Simpkins JW, Liu R (2015) Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: methylene blue connects the dots. Prog Neurobiol. doi:10.1016/j.pneurobio.2015.10.005
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. doi:10.2174/157015909787602823
Floyd RA, Carney JM (1992) Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol 32(Suppl):S22–S27
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647. doi:10.1016/j.tins.2009.08.002
Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT (2001) Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 21(4):374–384. doi:10.1097/00004647-200104000-00006
Urrutia PJ, Mena NP, Nunez MT (2014) The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol 5:38. doi:10.3389/fphar.2014.00038
Witte ME, Geurts JJ, de Vries HE, van der Valk P, van Horssen J (2010) Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10(5):411–418. doi:10.1016/j.mito.2010.05.014
Madden LK, DeVon HA (2015) A systematic review of the effects of body temperature on outcome after adult traumatic brain injury. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses 47(4):190–203. doi:10.1097/JNN.0000000000000142
Bayir H, Adelson PD, Wisniewski SR, Shore P, Lai Y, Brown D, Janesko-Feldman KL, Kagan VE et al (2009) Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Crit Care Med 37(2):689–695. doi:10.1097/CCM.0b013e318194abf2
Zhao M, Zhu P, Fujino M, Zhuang J, Guo H, Sheikh I, Zhao L, Li XK (2016) Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int J Mol Sci 17(12). doi:10.3390/ijms17122078
Toader AM, Filip A, Decea N, Muresan A (2013) Neuroprotective strategy in an experimental newborn rat model of brain ischemia and hypoxia: effects of resveratrol and hypothermia. Clujul medical 86(3):203–207
Shankaran S (2012) Therapeutic hypothermia for neonatal encephalopathy. Curr Treat Options Neurol 14(6):608–619. doi:10.1007/s11940-012-0200-y
Cai L, Thibodeau A, Peng C, Ji X, Rastogi R, Xin R, Singh S, Geng X et al (2016) Combination therapy of normobaric oxygen with hypothermia or ethanol modulates pyruvate dehydrogenase complex in thromboembolic cerebral ischemia. J Neurosci Res 94(8):749–758. doi:10.1002/jnr.23740
Briyal S, Gulati A (2010) Endothelin-A receptor antagonist BQ123 potentiates acetaminophen induced hypothermia and reduces infarction following focal cerebral ischemia in rats. Eur J Pharmacol 644(1–3):73–79. doi:10.1016/j.ejphar.2010.06.071
Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, Chen J (2007) Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Frontiers in bioscience : a journal and virtual library 12:1737–1747
Karibe H, Chen SF, Zarow GJ, Gafni J, Graham SH, Chan PH, Weinstein PR (1994) Mild intraischemic hypothermia suppresses consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res 649(1–2):12–18
Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS, Traystman RJ (2014) A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. J Neurosci Methods 222:34–41. doi:10.1016/j.jneumeth.2013.10.015
Ostadal P, Mlcek M, Kruger A, Horakova S, Skabradova M, Holy F, Svoboda T, Belohlavek J et al (2013) Mild therapeutic hypothermia is superior to controlled normothermia for the maintenance of blood pressure and cerebral oxygenation, prevention of organ damage and suppression of oxidative stress after cardiac arrest in a porcine model. J Transl Med 11:124. doi:10.1186/1479-5876-11-124
Guerra-Wallace MM, Casey FL 3rd, Bell MJ, Fink EL, Hickey RW (2013) Hyperoxia and hypoxia in children resuscitated from cardiac arrest. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 14(3):e143–e148. doi:10.1097/PCC.0b013e3182720440
Zhang H, Zhang JJ, Mei YW, Sun SG, Tong ET (2011) Effects of immediate and delayed mild hypothermia on endogenous antioxidant enzymes and energy metabolites following global cerebral ischemia. Chin Med J 124(17):2764–2766
Gong P, Hua R, Zhang Y, Zhao H, Tang Z, Mei X, Zhang M, Cui J et al (2013) Hypothermia-induced neuroprotection is associated with reduced mitochondrial membrane permeability in a swine model of cardiac arrest. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 33(6):928–934. doi:10.1038/jcbfm.2013.33
Ginimuge PR, Jyothi SD (2010) Methylene blue: revisited. J Anaesthesiol Clin Pharmacol 26(4):517–520
Ramirez Rivera J, Garayua JE (2006) Methemoglobinemia: life-threatening hazard of multiple drug ingestions. Boletin de la Asociacion Medica de Puerto Rico 98(2):118–121
Tardivo JP, Del Giglio A, de Oliveira CS, Gabrielli DS, Junqueira HC, Tada DB, Severino D, de Fatima TR et al (2005) Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications. Photodiagn Photodyn Ther 2(3):175–191. doi:10.1016/S1572-1000(05)00097-9
Gillman PK (2006) Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia 61(10):1013–1014. doi:10.1111/j.1365-2044.2006.04808.x
Vincer MJ, Allen AC, Evans JR, Nwaesei C, Stinson DA (1987) Methylene-blue-induced hemolytic anemia in a neonate. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 136(5):503–504
Acknowledgment
This study was supported by Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA; an American Heart Association Grant-in-Aid 15GRNT25240004; and a science and technology project of Xuzhou city, Jiangsu (KC15SH093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were approved by the institutional animal use committee and conformed to the local and international guidelines on the ethical use of animals.
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Lei Li and Rongli Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, L., Yang, R., Li, P. et al. Combination Treatment with Methylene Blue and Hypothermia in Global Cerebral Ischemia. Mol Neurobiol 55, 2042–2055 (2018). https://doi.org/10.1007/s12035-017-0470-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0470-1