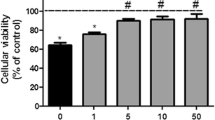
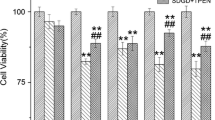
Abstract
Depletion of oxygen and glucose even for brief periods is sufficient to cause cerebral ischemia, which is a predominant worldwide cause of motor deficits with the reduction of life quality and subsequently death. Hence, more insights regarding protective measures against ischemic events are becoming a major research goal. Among the many neuronal factors, N-methyl-D-aspartate receptors (NMDAR), orexinergic neuroreceptors (ORXR), and sympatho-inhibitory neuropeptide catestatin (CST) are widely involved with ischemic episodes. In this study, it was possible to induce in vitro ischemic conditions of the hamster (Mesocricetus auratus) hippocampal and hypothalamic neuronal cultures, grown on a newly compartmentalized membrane system, via oxygen and glucose deprivation (OGD). These cultures displayed notably differentiated NMDARergic and ORXergic receptor expression activities along with evident brain-derived neurotrophic factor (BDNF) plus orexin A (ORX-A) secretion, especially under co-cultured conditions. Interestingly, addition of CST in OGD-insulted hippocampal cells accounted for upregulated GluN1 and ORX1R transcripts that in the case of the latter neuroreceptor was very strongly (p < 0.001) increased when co-cultured with hypothalamic cells. Similarly, hypothalamic neurons supplied very evident upregulations of GluN1, ORX1R, and above all of GluN2A transcripts along with increased BDNF and ORX-A secretion in the presence of hippocampal cells. Overall, the preferential CST effects on BDNF plus ORX-A production together with altered NMDAR and ORXR levels, especially in co-cultured hypothalamic cells pointed to ORX-containing neurons as major protective constituents against ischemic damages thus opening new scenarios on the cross-talking roles of CST during ischemic disorders.






Similar content being viewed by others
References
Iadecola C, Davisson RL (2008) Hypertension and cerebrovascular dysfunction. Cell Metab 7:476–484. doi:10.1016/j.cmet.2008.03.010
Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson RL, Iadecola C (2012) Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension 60:106–113. doi:10.1161/HYPERTENSIONAHA.112.193672
Chip S, Nitsch C, Wellmann S, Kapfhammer JP (2013) Subfield-specific neurovascular remodeling in the entorhino-hippocampal-organotypic slice culture as a response to oxygen-glucose deprivation and excitotoxic cell death. J Cereb Blood Flow Metab 33:508–518. doi:10.1038/jcbfm.2012.190
Alò R, Avolio E, Carelli A, Facciolo RM, Canonaco M (2011) Amygdalar glutamatergic neuronal systems play a key role on the hibernating state of hamsters. BMC Neurosci 12:10. doi:10.1186/1471-2202-12-10
Drew KL, Harris MB, LaManna JC, Smith MA, Zhu XW, Ma YL (2004) Hypoxia tolerance in mammalian heterotherms. J Exp Biol 207:3155–3162 . doi:10.1242/jeb.01114Review
Fontes MA, Xavier CH, Marins FR, Limborço-Filho M, Vaz GC, Müller-Ribeiro FC, Nalivaiko E (2014) Emotional stress and sympathetic activity: contribution of dorsomedial hypothalamus to cardiac arrhythmias. Brain Res 1554:49–58. doi:10.1016/j.brainres.2014.01.043
Brisson CD, Andrew RD (2012) A neuronal population in hypothalamus that dramatically resists acute ischemic injury compared to neocortex. J Neurophysiol 108:419–430. doi:10.1152/jn.00090.2012
Mele M, Avolio E, Alò R, Fazzari G, Mahata KS, Canonaco M (2014) Catestatin and orexin-a neuronal signals alter feeding habits in relation to hibernating states. Neuroscience 269:331–342. doi:10.1016/j.neuroscience.2014.03.065
Avolio E, Mahata SK, Mantuano E, Mele M, Alò R, Facciolo RM, Talani G, Canonaco M (2014) Antihypertensive and neuroprotective effects of catestatin and muscimol in spontaneously hypertensive rats: interaction with GABAergic transmission in amygdala and brainstem. Neuroscience 270:48–57. doi:10.1016/j.neuroscience.2014.04.001
Mele M, Alò R, Avolio E, Zizza M, Fazzari G, Canonaco M (2015) AMPAergic mechanisms linked to cerebral ischemia. Therapeutic target for Neurological Diseases 2:1. doi:10.14800/ttnd.478
Gascón S, Deogracias R, Sobrado M, Roda JM, Renart J, Rodríguez-Peña A, Díaz-Guerra M (2005) Transcription of the NR1 subunit of the N-methyl-D-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. J Biol Chem 280:35018–35027. doi:10.1074/jbc.M504108200
Furukawa H, Singh SK, Mancusso R, Gouaux E (2005) Subunit arrangement and function in NMDA receptors. Nature 438:185–192. doi:10.1038/nature04089
Tsujino N, Sakurai T (2013) Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 7:28. doi:10.3389/fnbeh.2013.00028
Nakamachi T, Endo S, Ohtaki H, Yin L, Kenji D, Kudo Y, Funahashi H, Matsuda K et al (2005) Orexin-1 receptor expression after global ischemia in mice. Regul Pept 126:49–54. doi:10.1016/j.regpep.2004.08.037
Xiong X, White RE, Xu L, Yang L, Sun X, Zou B, Pascual C, Sakurai T et al (2013) Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke 44:764–770. doi:10.1161/STROKEAHA.112.681700
Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, O’Connor DT (2003) Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J Biol Chem 278:32058–32067. doi:10.1074/jbc.M305545200
Penna C, Tullio F, Perrelli MG, Mancardi D, Pagliaro P (2012) Cardioprotection against ischemia/reperfusion injury and chromogranin A-derived peptides. Curr Med Chem 19:4074–4085. doi:10.2174/092986712802429966
De Bartolo L, Rende M, Morelli S, Giusi G, Salerno S, Piscioneri A, Giordano A, Di Vito A et al (2008) Influence of membrane surface properties on the growth of neuronal cells isolated form hippocampus. J Mem Sci 325:139–149. doi:10.1002/term.434
Giusi G, Facciolo RM, Rende M, Alo R, Di Vito A, Salerno S, Morelli S, De Bartolo L et al (2009) Distinct α subunits of the GABAA receptor are responsible for early hippocampal silent neuron-related activities. Hippocampus 19:1103–1114. doi:10.1002/hipo.20584
Morelli S, Piscioneri A, Salerno S, Tasselli F, Di Vito A, Giusi G, Canonaco M, Drioli E et al (2012) PAN hollow fiber membranes elicit functional hippocampal neuronal network. J Mater Sci Mater Med 23:149–156. doi:10.1007/s10856-011-4484-3
Morelli S, Piscioneri A, Salerno S, Rende M, Campana C, Tasselli F, Di Vito A, Giusi G et al (2012) Flat and tubular membrane systems for the reconstruction of hippocampal neuronal network. J Tissue Eng Regen Med 6:299–313. doi:10.1002/term.434
Di Vito A, Mele M, Piscioneri A, Morelli S, De Bartolo L, Barni T, Facciolo RM (2014) Overstimulation of glutamate signals leads to hippocampal transcriptional plasticity in hamsters. Cell Mol Neurobiol 34:501–509. doi:10.1007/s10571-014-0034-0
Piscioneri A, Morelli S, Mele M, Canonaco M, Bilotta E, Pantano P, Drioli E, De Bartolo L (2015) Neuroprotective effect of human mesenchymal stem cells in a compartmentalized neuronal membrane system. Acta Biomater 24:297–308. doi:10.1016/j.actbio.2015.06.013
Nuñez A, Rodrigo-Angulo ML, Andrés ID, Garzón M (2009) Hypocretin/orexin neuropeptides: participation in the control of sleep-wakefulness cycle and energy homeostasis. Curr Neuropharmacol 7:50–59. doi:10.2174/157015909787602797
Wolk DA, Detre JA (2012) Arterial spin labeling MRI: an emerging biomarker for Alzheimer’s disease and other neurodegenerative conditions. Curr Opin Neurol 25:421–428. doi:10.1097/WCO.0b013e328354ff0a
Takeda S, Sato N, Morishita R (2014) Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 6:171. doi:10.3389/fnagi.2014.00171
De Bartolo L, Rende M, Giusi G, Morelli S, Piscioneri A, Canonaco M, Orioli E (2007) Membrane bio-hybrid systems: a valuable tool for the study of neuronal activities. In: Canonaco M, Facciolo RM (eds) Evolutionary molecular strategies and plasticity. Research Signpost Press, Kerala, pp. 379–396
Batista Lobo S, Denyer M, Britland S, Javid FA (2007) Development of an intestinal cell culture model to obtain smooth muscle cells and myenteric neurones. J Anat 211:819–829
Morelli S, Piscioneri A, Messina A, Salerno S, Al-Fageeh MB, Drioli E, De Bartolo L (2015) Neuronal growth and differentiation on biodegradable membranes. J. Tissue Eng Regen Med 9:106–117. doi:10.1002/term.1618
Vogt AK, Wrobel G, Meyer W, Knoll W, Offenhäusser A (2005) Synaptic plasticity in micropatterned neuronal networks. doi:10.1016/j.biomaterials.2004.07.031
Farso MC, Carroll FY, Beart PM (2006) Establishment of primary cultures of rat olfactory bulb under serum-free conditions for studies of cellular injury. Cell Tissue Res 32:343–349. doi:10.1007/s00441-005-0056-5
Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S et al (2010) The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res 107:1326–1335. doi:10.1161/CIRCRESAHA.110.219493
Lushnikova IV, Voronin KY, Malyarevskyy PY, Skibo GG (2004) Morphological and functional changes in rat hippocampal lice cultures after short-term oxygen-glucose deprivation. J Cell Mol Med 8:241–248. doi:10.1111/j.1582-4934.2004.tb00279.x
Brooks-Kayal AR, Jin H, Price M, Dichter MA (1998) Developmental expression of GABAA receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. J Neurochem 70:1017–1028. doi:10.1046/j.1471-4159.1998.70031017.x
Dos-Anjos S, Martínez-Villayandre B, Montori S, Pérez-García CC, Fernández-López A (2009) Early modifications in N-methyl-D-aspartate receptor subunit mRNA levels in an oxygen and glucose deprivation model using rat hippocampal brain slices. Neuroscience 164:1119–1126. doi:10.1016/j.neuroscience.2009.09.019
Neuhaus W, Burek M, Djuzenova CS, Thal SC, Koepsell H, Roewer N, Förster CY (2012) Addition of NMDA-receptor antagonist MK801 during oxygen/glucose deprivation moderately attenuates the upregulation of glucose uptake after subsequent reoxygenation in brain endothelial cells. Neurosci Lett 506:44–49. doi:10.1016/j.neulet.2011.10.045
Zhou Y, Li HL, Zhao R, Yang LT, Dong Y, Yue X, Ma YY, Wang Z et al (2010) Astrocytes express N-methyl-D-aspartate receptor subunits in development, ischemia and post-ischemia. Neurochem Res 35:2124–2134. doi:10.1007/s11064-010-0325-x
Yu L, Wang N, Zhang Y, Wang Y, Li J, Wu Q, Liu Y (2014) Neuroprotective effect of muscone on glutamate-induced apoptosis in PC12 cells via antioxidant and Ca(2+) antagonism. Neurochem Int 70:10–21. doi:10.1016/j.neuint.2014.03.003
Chen YW, Lin MF, Chen YC, Hung CH, Tzeng JI, Wang JJ (2013) Exercise training attenuates postoperative pain and expression of cytokines and N-methyl-D-aspartate receptor subunit 1 in rats. Reg Anesth Pain Med 38:282–288. doi:10.1097/AAP.0b013e31828df3f9
De Montigny A, Elhiri I, Allyson J, Cyr M, Massicotte G (2013) NMDA reduces tau phosphorylation in rat hippocampal slices by targeting NR2A receptors, GSK3β, and PKC activities. Neural Plast 2013:261593. doi:10.1155/2013/261593
Vizi ES, Kisfali M, Lőrincz T (2013) Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects. Brain Res Bull 93:32–38. doi:10.1016/j.brainresbull.2012.10.005
Zhang X, Zhang Q, Tu J, Zhu Y, Yang F, Liu B, Brann D, Wang R (2015) Prosurvival NMDA 2A receptor signaling mediates postconditioning neuroprotection in the hippocampus. Hippocampus 25:286–296. doi:10.1002/hipo.22372
Li X, Zhang YY, Chen ZQ, Jiang ZL, Sun L, Xu LH, Yang Y, Zhang YF (2014) D-serine-induced inactivation of NMDA receptors in cultured rat hippocampal neurons expressing NR2A subunits is Ca2+-dependent. CNS Neurosci Ther 20:951–960. doi:10.1111/cns.12308
Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Fend XY, Xu L, Duan WH et al (2008) Differential roles of NMDA receptors subypes in ishemic neuronal cell death and ischemic tolerance. Stroke 39:3042–3048. doi:10.1161/STROKEHA.108.521898
Irving EA, Harrison DC, Babbs AJ, Mayes AC, Campbell CA, Hunter AJ, Upton N, Parsons AA (2002) Increased cortical expression of the orexin-1 receptor following permanent middle cerebral artery occlusion in the rat. Neurosci Lett 324:53–56. doi:10.1016/S0304-3940(02)00176-3
Sikder D, Kodadek T (2007) The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev 21:2995–3005. doi:10.1101/gad.1584307
Mayr M, May D, Gordon O, Madhu B, Gilon D, Yin X, Xing Q, Drozdov I et al (2011) Metabolic homeostasis is maintained in myocardial hibernation by adaptive changes in the transcriptome and proteome. Mol Cell Cardiol 50:982–990. doi:10.1016/j.yjmcc.2011.02.010
Sokołowska P, Urbańska A, Biegańska K, Wagner W, Ciszewski W, Namiecińska M, Zawilska JB (2014) Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. J Mol Neurosci 52:48–55. doi:10.1007/s12031-013-0165-7
Kukko-Lukjanov TK, Soini S, Taira T, Michelsen KA, Panula P, Holopainen IE (2006) Histaminergic neurons protect the developing hippocampus from kainic acid-induced neuronal damage in an organotypic coculture system. J Neurosci 26:1088–1097
Beck B, Pourié G (2013) Ghrelin, neuropeptide Y, and other feeding-regulatory peptides active in the hippocampus: role in learning and memory. Nutr Rev 71:541–561
Yang S, Zhou G, Liu H, Zhang B, Li J, Cui R, Du Y (2013) Protective effects of p38 MAPK inhibitor SB202190 against hippocampal apoptosis and spatial learning and memory deficits in a rat model of vascular dementia. Biomed Res Int 2013:215798. doi:10.1155/2013/215798
Alvarez VA, Ridenour DA, Sabatini BL (2007) Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. J Neurosci 27:7365–7376. doi:10.1523/JNEUROSCI.0956-07.2007
Hu S, Cui W, Mak S, Xu D, Hu Y, Tang J, Choi C, Lee M et al (2015) Substantial neuroprotective and neurite outgrowth-promoting activities by Bis(propyl)-cognitin via the activation of Alpha7-nAChR, a promising anti-Alzheimer’s dimer. ACSChem Neurosci 6:1536–1545. doi:10.1021/acschemneuro.5b00108
Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA (2015) Role of orexin a signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett 606:140–144. doi:10.1016/j.neulet.2015.08.033
Brisson CD, Lukewich MK, Andrew RD (2013) A distinct boundary between the higher brain’s susceptibility to ischemia and the lower brain’s resistance. PLoS One 8:e79589. doi:10.1371/journal.pone.0079589
Shahid IZ, Rahman AA, Pilowsky PM (2012) Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol 165:2292–2303. doi:10.1111/j.1476-5381.2011.01694.x
Gaede AH, Pilowsky PM (2010) Catestatin in rat RVLM is sympathoexcitatory, increases barosensitivity, and attenuates chemosensitivity and the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 299:1538–1545. doi:10.1152/ajpregu.00335.2010
Gaede AH, Pilowsky PM (2012) Catestatin, a chromogranin A-derived peptide, is sympathoinhibitory and attenuates sympathetic barosensitivity and the chemoreflex in rat CVLM. Am J Physiol Regul Integr Comp Physiol 302:365–372. doi:10.1152/ajpregu.00409.2011
Sahu BS, Mohan J, Sahu G, Singh PK, Sonawane PJ, Sasi BK, Allu PK, Maji SK et al (2012) Molecular interactions of the physiological anti-hypertensive peptide catestatin with the neuronal nicotinic acetylcholine receptor. J Cell Sci 125:2323–2337. doi:10.1242/jcs.103176
Mele M, Canonaco M (2014) Catestatin and orexin-A influence hamster thermic states during hibernation. Temperature 1:24–25. doi:10.4161/temp.29547
Lim JY, Reighard CP, Crowther DC (2015) The pro-domains of neurotrophins, including BDNF, are linked to Alzheimer’s disease through a toxic synergy with Aβ. Hum Mol Genet 24:3929–3938. doi:10.1093/hmg/ddv130
Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE (2006) Prenatal stress and neonatal rat brain development. Neuroscience 137:145–155
Mariga A, Zavadil J, Ginsberg SD, Chao MV (2015) Withdrawal of BDNF from hippocampal cultures leads to changes in genes involved in synaptic function. Dev Neurobiol 75:173–192. doi:10.1002/dneu.22216
He YY, Zhang XY, Yung WH, Zhu JN, Wang JJ (2013) Role of BDNF in central motor structures and motor diseases. Mol Neurobiol 48:783–793. doi:10.1007/s12035-013-8466-y
Takada Y, Beyer LA, Swiderski DL, O’Neal AL, Prieskorn DM, Shivatzki S, Avraham KB, Raphael Y (2014) Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy. Hear Res 309:124–135. doi:10.1016/j.heares.2013.11.009
Wang Y, Li J, Kong P, Zhao S, Yang H, Chen C, Yan J (2015) Enhanced expression of neurotrophic factors in the injured spinal cord through vaccination with myelin basic protein-derived peptide pulsed dendritic cells. Spine (Phila Pa 1976) 40:95–101. doi:10.1097/BRS.0000000000000694
Laurén HB, Ruohonen S, Kukko-Lukjanov TK, Virta JE, Grönman M, Lopez-Picon FR, Järvelä JT et al (2013) Status epilepticus alters neurogenesis and decreases the number of GABAergic neurons in the septal dentate gyrus of 9-day-old rats at the early phase of epileptogenesis. Brain Res 1516:33–44. doi:10.1016/j.brainres.2013.04.028
Morelli S, Piscioneri A, Salerno S, Al-Fageeh M, Drioli E, De Bartolo L (2014) Neuroprotective effect of didymin on H2O2-induced injury in neuronal membrane system. Cells Tissues Organs 199:184–200. doi:10.1159/000365072
Yang W, Zheng H, Wang Y, Lian F, Hu Z, Xue S (2015) Nesprin-1 has key roles in the process of mesenchymal stem cell differentiation into cardiomyocyte-like cells in vivo and in vitro. Mol Med Rep 11:133–142. doi:10.3892/mmr.2014.2754
Song MS, Learman CR, Ahn KC, Baker GB, Kippe J, Field EM, Dunbar GL (2015) In vitro validation of effects of BDNF-expressing mesenchymal stem cells on neurodegeneration in primary cultured neurons of APP/PS1 mice. Neuroscience 307:37–50. doi:10.1016/j.neuroscience.2015.08.011
Su M, Hong J, Zhao Y, Liu S, Xue X (2015) MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA 132 in rats with depression. Mol Med Rep 12:5399–5406. doi:10.3892/mmr.2015.4104
Acknowledgments
Italian University Research Ministry (MIUR) and Calabrian Region (POR, FSE-2007/2013) have funded this work. All authors declare that this original paper has not been published previously as well as not having any conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mele, M., Morelli, S., Fazzari, G. et al. Application of the Co-culture Membrane System Pointed to a Protective Role of Catestatin on Hippocampal Plus Hypothalamic Neurons Exposed to Oxygen and Glucose Deprivation. Mol Neurobiol 54, 7369–7381 (2017). https://doi.org/10.1007/s12035-016-0240-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0240-5