Abstract
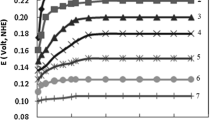
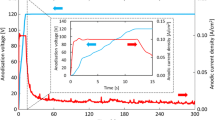
The corrosion behaviour of antimony (Sb) and the stability of the anodic-generated coating on its surface were studied in a 4.97 M H2SO4 solution using cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), potentiodynamic polarization, scanning electron microscopy (SEM) and atomic force microscopy (AFM). According to the CV data, the current plateau value increases as the scan rate increases, indicating that anodic layer development on the Sb surface occurs via a low-field migration mechanism. The EIS results confirmed that the pre-immersion oxide film and the anodic-produced layer on Sb are liable to dissolution in concentrated acid solution. The polarization results revealed that when the temperature rises, the rate of Sb corrosion in the acid solution increases. SEM and AFM surface investigation of the anodic layer generated on the antimony surface revealed that it is homogenous throughout its extension on the surface and lacks pores. For the corrosion of Sb in concentrated acid solution, the thermodynamic parameters Ea (activation energy), ΔH (enthalpy change) and ΔS (entropy change) were calculated.










Similar content being viewed by others
References
Brady G S 1971 Handbook of materials (New York: McGraw Hills Book Co.) p 64
Take T and Akuto K 1988 Electr. Commun. 36 5
Mukerjee D and Guruviah S 1988 Key Eng. Mater. 20 20
El-Basiouny M S, Hefny M M and Mogoda A S 1984 Annali di Chimica 74 729
El-Basiouny M S, Hefny M M and Mogoda A S 1985 Corrosion 41 611
Hefny M M, Badawy W A, Mogoda A S and El-Basiouny M S 1985 Electrochim. Acta 30 1017
Badawy W A, Mogoda A S and Ibrahim M M 1988 Electrochim. Acta 33 1367
Gadallh A G, Salih S A, Hefny M M and Mogoda A S 1990 Corrosion 46 214
Mogoda A S, Badawy W A and Ibrahim M M 1995 Bull. Electrochem. 11 281
Mogoda A S, Badawy W A and Ibrahim M M 1995 Indian J. Chem. Technol. 2 217
Mogoda A S 2001 Thin Solid Films 394 174
Mogoda A S and Abd El-Haleem T M 2003 Corrosion 59 3
Mogoda A S and Abd El-Haleem T M 2003 Thin Solid Films 441 6
Laitinen T, Salmi K, Sundholm G, Morahovt B and Pavlovt D 1991 Electrochim. Acta 36 605
Pavlov D, Monahov B, Sundholm G and Laitinen T 1991 J. Electroanal. Chem. 305 57
Monahov B and Pavlov D 1994 J. Electrochem. Soc. 141 2316
Brinic S, Metikos-Hukovic M and Babic R 1995 J. Power Sources 55 19
Sun Q and Guo Y 2000 J. Electroanal. Chem. 493 123
Rocca E and Steinmetz J 2003 J. Electroanal. Chem. 543 153
El-Nowihy G H and El-Deab M S 2022 J. Electrochem. Soc. 169 046508
Mogoda A S and Farag A R 2022 Silicon. https://doi.org/10.1007/s12633-022-01861-x
Mogoda A S, Zohdy K M and Aboutabl M A 2022 Silicon 14 2573
Mogoda A S and Zohdy K M 2022 Int. J. Electrochem. Sci. https://doi.org/10.20964/2022.01.23
Bojinov M and Pavlov D 1991 J. Electroanal. Chem. 315 201
Pavlov D, Bojinov M, Laitinen T and Sundholm G 1991 Electrochim. Acta 36 2081
Pavlov D, Bojinov M, Laitinen T and Sundholm G 1991 Electrochim. Acta 36 2087
Metikos-Hukovic M, Babic R and Omanovic S 1994 J. Electroanal. Chem. 374 199
Metikos-Hukovic M, Babic R and Brinic S 2006 J. Power Sources 157 563
Williams D and Wright G A 1976 Electrochim. Acta 21 1009
Bojinov M, Kanazirski I and Girginov A 1995 Electrochim. Acta 40 873
El-Taib Heakal F, Ghoneim A A, Mogoda A S and Awad Kh A 2011 Corros. Sci. 53 2728
Ghoneim A A, Mogoda A S, Awad Kh A and El-Taib Heakal F 2012 Int. J. Electrochem. Sci. 7 6539
Ghoneim A A, El-Taib Heakal F, Mogoda A S and Awad Kh A 2010 Surf. Interface Anal. 42 1695
Juttner K 1990 Electrochim. Acta 35 1501
Rammelt U and Reinhard G 1990 Electrochim. Acta 35 1045
Mogoda A S and Zohdy K M 2020 Int. J. Electrochem. Sci. 15 8070
Atkins P W 1998 Physical Chemistry (Oxford University Press) p 864
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mogoda, A.S., Zohdy, K.M. EIS characteristics of antimony and its anodic oxide film in sulphuric acid solution. Bull Mater Sci 45, 181 (2022). https://doi.org/10.1007/s12034-022-02771-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-022-02771-9