Abstract
Esterases are hydrolases that contribute to the hydrolysis of ester bonds into both water-soluble acyl esters and emulsified glycerol-esters containing short-chain acyl groups. They have garnered significant attention from biotechnologists and organic chemists due to their immense commercial value. Esterases, with their diverse and significant properties, have become highly sought after for various industrial applications. Synthesized ubiquitously by a wide range of living organisms, including animals, plants, and microorganisms, these enzymes have found microbial esterases to be the preferred choice in industrial settings. The cost-effective production of microbial esterases ensures higher yields, unaffected by seasonal variations. Their applications span diverse sectors, such as food manufacturing, leather tanneries, paper and pulp production, textiles, detergents, cosmetics, pharmaceuticals, biodiesel synthesis, bioremediation, and waste treatment. As the global trend shifts toward eco-friendly and sustainable practices, industrial processes are evolving with reduced waste generation, lower energy consumption, and the utilization of biocatalysts derived from renewable and unconventional raw materials. This review explores the background, structural characteristics, thermostability, and multifaceted roles of bacterial esterases in crucial industries, aiming to optimize and analyze their properties for continued successful utilization in diverse industrial processes. Additionally, recent advancements in esterase research are overviewed, showcasing novel techniques, innovations, and promising areas for further exploration.
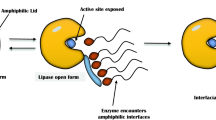
Graphical Abstract




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article [and there is no supplementary information file].
References
Akil, E., Pereira, A. D. S., El-Bacha, T., Amaral, P. F., & Torres, A. G. (2020). Efficient production of bioactive structured lipids by fast acidolysis catalyzed by Yarrowia lipolytica lipase, free and immobilized in chitosan-alginate beads, in solvent-free medium. International Journal of Biological Macromolecules, 163, 910–918. https://doi.org/10.1016/j.ijbiomac.2020.06.282
Alvarez, Y., Esteban-Torres, M., Cortés-Cabrera, Á., Gago, F., Acebron, I., Benavente, R., Mardo, K., De Las Rivas, B., Muñoz, R., & Mancheño, J. M. (2014). Esterase LpEst1 from Lactobacillus plantarum: A novel and atypical member of the αβ hydrolase superfamily of enzymes. PLoS ONE, 9(3), e92257. https://doi.org/10.1371/journal.pone.0092257
Alvarez-Macarie, E., & Baratti, J. (2000). short chain flavour ester synthesis by a new esterase from Bacillus licheniformis. Journal of Molecular Catalysis B Enzymatic, 10, 377–383. https://doi.org/10.1016/S1381-1177(99)00109-5
Ammar, E. E. (2022). Environmental impact of biodegradation (pp. 1–40). Springer. https://doi.org/10.1007/978-3-030-83783-9_27-1
Andrada, E., Marquez, A., Dib, E. P. C., Gauffin-Cano, P., & Medina, R. (2023). Corn stover silage inoculated with ferulic acid esterase producing L. johnsonii, L. plantarum, L. fermentum, and L. brevis strains fermentative and nutritional parameters. Fermentation, 9(4), 331. https://doi.org/10.3390/fermentation9040331
Aquino, M., Saparrat, M. C. N., & Pildain, M. B. (2023). Umbelopsis (Mucoromycota) from patagonia, argentina: Identification, phylogenetic analysis, and expression profiling of lipase activity and lipid accumulation in selected isolates. Mycological Progress, 22(3), 18. https://doi.org/10.1007/s11557-023-01866-9
Ashenhurst, J. (2022). Transesterification. Master Organic Chemistry. https://www.masterorganicchemistry.com/2022/11/10/transesterification/
Ballinas-Casarrubias, L., Sánchez, G. G., Eguiarte-Franco, S., Siqueiros-Cendón, T., Flores-Gallardo, S. G., Villa, E. D., De Dios Hernández, M., Rocha-Gutiérrez, B. A., & Rascón-Cruz, Q. (2020). Chemical characterization and enzymatic control of stickies in kraft paper production. Polymers Journal, 12(1), 245. https://doi.org/10.3390/polym12010245
Bartha-Vári, J., Moisă, M. E., Bencze, L., Irimie, F. D., Paizs, C., & Toşa, M. I. (2020). Efficient biodiesel production catalyzed by nanobioconjugate of lipase from Pseudomonas fluorescens. Molecules, 25(3), 651. https://doi.org/10.3390/molecules25030651
Berry, S., Bruce, J., Steenson, S., Stanner, S., Buttriss, J., Spiro, A., Gibson, P., Bowler, I., Dionisi, F., Farrell, L., Glass, A., Lovegrove, J. A., Nicholas, J., Peacock, E., Porter, S., Mensink, R. P., & Hall, W. L. (2019). Interesterified fats: What are they and why are they used? A briefing report from the Roundtable on interesterified fats in foods. Nutrition Bulletin, 44(4), 363–380. https://doi.org/10.1111/nbu.12397
Bhardwaj, K. K., Dogra, A., Kapoor, S., Mehta, A., & Gupta, R. (2020). Purification and properties of an esterase from Bacillus licheniformis and it’s application in synthesis of octyl acetate. Open Microbiology Journal, 14(1), 113–121. https://doi.org/10.2174/1874285802014010113
Bhardwaj, K. K., Kishen, S., Mehta, A., Sharma, A., & Gupta, R. (2021). Purification of high molecular weight thermotolerant esterase from Serratia sp. and its characterization. Biotech, 11(6), 308. https://doi.org/10.1007/s13205-021-02852-2
Bhatt, P., Bhatt, K., Huang, Y., Lin, Z., & Chen, S. (2020). Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere, 244, 125507. https://doi.org/10.1016/j.chemosphere.2019.125507
Bhatt, P., Zhou, X., Huang, Y., Zhang, W., & Chen, S. (2021). Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. Journal of Hazardous Materials, 411, 125026. https://doi.org/10.1016/j.jhazmat.2020.125026
Bibra, M., Kunreddy, V. R., & Sani, R. K. (2018). Thermostable xylanase production by Geobacillus sp. strain DUSELR13 and its application in ethanol production with lignocellulosic biomass. Microorganisms, 6(3), 93. https://doi.org/10.3390/microorganisms6030093
Bleffert, F., Granzin, J., Gohlke, H., Batra-Safferling, R., Jaeger, K. E., & Kovacic, F. (2019). Pseudomonas aeruginosaesterase PA2949, a bacterial homolog of the human membrane esterase ABHD6: Expression, purification and crystallization. Acta Crystallographica Section F: Structural Biology, 75(4), 270–277. https://doi.org/10.1107/s2053230x19002152
Boczar, B. A., Forney, L. J., Begley, W. M., Larson, R. J., & Federle, T. W. (2001). Characterization and distribution of esterase activity in activated sludge. Water Research, 35(17), 4208–4216. https://doi.org/10.1016/s0043-1354(01)00150-6
Braddick, H., Tipping, W. J., Wilson, L. T., Jaconelli, H. S., Grant, E. K., Faulds, K., Graham, D., & Tomkinson, N. C. O. (2023). Determination of intracellular esterase activity using ratiometric raman sensing and spectral phasor analysis. Analytical Chemistry, 95(12), 5369–5376. https://doi.org/10.1021/acs.analchem.2c05708
Bulgari, D., Renzetti, S., Messgo-Moumene, S., Monti, E., & Gobbi, E. (2023). Optimization of esterase production in solid-state fermentation of agricultural digestate. Fermentation, 9(6), 524. https://doi.org/10.3390/fermentation9060524
Carr, P., & Ollis, D. L. (2009). Hydrolase fold: An update. Protein and Peptide Letters, 16(10), 1137–1148. https://doi.org/10.2174/092986609789071298
Chahinian, H., & Sarda, L. (2009). Distinction between esterases and lipases: Comparative biochemical properties of sequence-related carboxylesterases. Protein and Peptide Letters, 16(10), 1149–1161. https://doi.org/10.2174/092986609789071333
Chatterjee, A., Puri, S., Sharma, P., Deepa, P. R., & Chowdhury, S. (2023). Nature-inspired enzyme engineering and sustainable catalysis: Biochemical clues from the world of plants and extremophiles. Frontiers Bioengineering Biotechnology, 11, 1229300. https://doi.org/10.3389/fbioe.2023.1229300
Chávez, R., Schachter, K., Navarro, C., Peirano, A., Bull, P., & Eyzaguirre, J. (2004). The acetyl xylan esterase II gene from Penicilliumpurpurogenum is differentially expressed in several carbon sources, and tightly regulated by pH. Biological Research, 37(1), 107. https://doi.org/10.4067/s0716-97602004000100011
Chen, Y., Black, D. S., & Reilly, P. J. (2016). Carboxylic ester hydrolases: Classification and database derived from their primary, secondary and tertiary structures. Protein Science, 25(11), 1942–1953. https://doi.org/10.1002/pro.3016
D’Errico, C., Börjesson, J., Ding, H., Krogh, K. B. R. M., Spodsberg, N., Madsen, R., & Monrad, R. N. (2016). Improved biomass degradation using fungal glucuronoyl—esterases—hydrolysis of natural corn fiber substrate. Journal of Biotechnology, 219, 117–123. https://doi.org/10.1016/j.jbiotec.2015.12.024
Dahiya, D., & Nigam, P. S. (2022). Sustainable biosynthesis of esterase enzymes of desired characteristics of catalysis for pharmaceutical and food industry employing specific strains of microorganisms. Sustainability, 14(14), 8673. https://doi.org/10.3390/su14148673
Darvishi, F., Fathi, Z., Ariana, M., & Moradi, H. (2017). Yarrowia lipolytica as a workhorse for biofuel production. Biochemical Engineering Journal, 127, 87–96. https://doi.org/10.1016/j.bej.2017.08.013
Dash, A., & Sahoo, S. K. (2021). Role of enzymes in textile processing (pp. 395–410). Springer. https://doi.org/10.1007/978-981-33-4195-1_19
De Waele, S., Vandenberghe, I., Laukens, B., Planckaert, S., Verweire, S., Van Bogaert, I., Soetaert, W., Devreese, B., & Ciesielska, K. (2018). Optimized expression of the Starmerella bombicola lactone esterase in Pichia pastoris through temperature adaptation, codon-optimization and co-expression with HAC1. Protein Expression and Purification, 143, 62–70. https://doi.org/10.1016/j.pep.2017.10.016
Di, L. (2019). The impact of carboxylesterases in drug metabolism and pharmacokinetics. Current Drug Metabolism, 20(2), 91–102. https://doi.org/10.2174/1389200219666180821094502
Dilokpimol, A., Makela, M., Mansouri, S., Бeлoвa, O. A., Waterstraat, M., Bunzel, M., De Vries, R. P., & Hildén, K. (2017). Expanding the feruloyl esterase gene family of Aspergillus niger by characterization of a feruloyl esterase, FaeC. New Biotechnology, 37, 200–209. https://doi.org/10.1016/j.nbt.2017.02.007
Distaso, M. A., Cea-Rama, I., Coscolín, C., Chernikova, T. N., Tran, H., Ferrer, M., Sanz-Aparicio, J., & Golyshin, P. N. (2023). The mobility of the cap domain is essential for the substrate promiscuity of a family IV esterase from sorghum rhizosphere microbiome. Applied Environment Microbiology, 89(1), e01807. https://doi.org/10.1128/aem.01807-22
Dong, H., Pang, L., Cong, H., Shen, Y., & Yu, B. (2019). Application and design of esterase-responsive nanoparticles for cancer therapy. Journal Drug Delivery, 26(1), 416–432. https://doi.org/10.1080/10717544.2019.1588424
Doraiswamy, N., Sarathi, M., & Pennathur, G. (2020). Improvement in biochemical characteristics of cross-linked enzyme aggregates (CLEAs) with magnetic nanoparticles as support matrix. Methods in Enzymology, 630, 133–158. https://doi.org/10.1016/bs.mie.2019.10.019
Duan, X., Dai, Y., & Zhang, T. (2021). Characterization of feruloyl esterase from bacillus pumilus SK52.001 and its application in ferulic acid production from de-starched wheat bran. Foods, 10(6), 1229. https://doi.org/10.3390/foods10061229
Esteban-Torres, M., Mancheño, J. M., De Las Rivas, B., & Múñoz, R. (2014). Production and characterization of a tributyrin esterase from Lactobacillus plantarum suitable for cheese lipolysis. Journal of Dairy Science, 97(11), 6737–6744. https://doi.org/10.3168/jds.2014-8234
Fasim, A., More, V. S., & More, S. S. (2021). Large-scale production of enzymes for biotechnology uses. Current Opinion in Biotechnology, 69, 68–76. https://doi.org/10.1016/j.copbio.2020.12.002
Finch, A. J., & Kim, J. R. (2018). Thermophilic proteins as versatile scaffolds for protein engineering. Microorganisms, 6(4), 97. https://doi.org/10.3390/microorganisms6040097
Frederick, J., Hennessy, F., Horn, U., De La Torre Cortés, P., Van Den Broek, M., Strych, U., Willson, R. C., Hefer, C. A., Daran, J., Sewell, B. T., Otten, L. G., & Brady, D. (2020). The complete genome sequence of the nitrile biocatalyst Rhodococcus rhodochrous ATCC BAA-870. BMC Genomics, 21(1), 1. https://doi.org/10.1186/s12864-019-6405-7
Gao, X., Mao, X., Lu, P., Secundo, F., Xue, C., & Sun, J. (2019). Cloning, expression, and characterization of a novel thermostable and alkaline-stable esterase from Stenotrophomonas maltophilia OUC_Est10 catalytically active in organic solvents. Catalysts, 9(5), 401. https://doi.org/10.3390/catal9050401
García-Calvo, L., Rodríguez-Castro, R., Ullán, R. V., Albillos, S. M., Fernández-Aguado, M., Vicente, C. M., Degnes, K. F., Sletta, H., & Barreiro, C. (2023). Penicillium chrysogenum as a fungal factory for feruloyl esterases. Applied Microbiology and Biotechnology, 107(2–3), 691–717. https://doi.org/10.1007/s00253-022-12335-w
Gil-Rivas, A., De Pascual-Teresa, B., Ortín, I., & Ramos, A. (2023). New advances in the exploration of esterases with PET and fluorescent probes. Molecules, 28(17), 6265. https://doi.org/10.3390/molecules28176265
Godinho, L. F., Reis, C. R., Tepper, P. G., Poelarends, G. J., & Quax, W. J. (2011). Discovery of an Escherichia coli esterase with high activity and enantioselectivity toward 1,2- O -isopropylideneglycerol esters. Applied and Environment Microbiology, 77(17), 6094–6099. https://doi.org/10.1128/aem.05122-11
Goyal, A., Vaish, D. C., Agrawal, R., Choudhary, S., & Nayak, R. (2022). Sustainable manufacturing through systematic reduction in cycle time. Sustainability, 14(24), 16473. https://doi.org/10.3390/su142416473
Grizanova, E. V., Кpыцынa, T. И, Surcova, V., & Dubovskiy, I. M. (2019). The role of midgut nonspecific esterase in the susceptibility of Galleria mellonella larvae to Bacillus thuringiensis. Journal of Invertebrate Pathology, 166, 107208. https://doi.org/10.1016/j.jip.2019.107208
Haldar, D., Sen, D., & Gayen, K. (2016). A review on the production of fermentable sugars from lignocellulosic biomass through conventional and enzymatic route—a comparison. International Journal of Green Energy, 13(12), 1232–1253. https://doi.org/10.1080/15435075.2016.1181075
Hassan, T. U., & Bano, A. (2016). Comparative effects of wild type Stenotrophomonasmaltophilia and its indole acetic acid-deficient mutants on wheat. Plant Biology, 18(5), 835–841. https://doi.org/10.1111/plb.12477
He, H., Yu, Q., Ding, Z., Zhang, L., Shi, G., & Li, Y. (2023). Biotechnological and food synthetic biology potential of platform strain: Bacillus licheniformis. Synthetic and Systems Biotechnology, 8(2), 281–291. https://doi.org/10.1016/j.synbio.2023.03.008
Hetrick, K. J., & Raines, R. T. (2022). Assessing and utilizing esterase specificity in antimicrobial prodrug development. Methods in Enzymology, 664, 199–220. https://doi.org/10.1016/bs.mie.2021.11.008
Holland, R., Liu, S., Crow, V. L., Delabre, M., Lubbers, M. W., Bennett, M. J., & Norris, G. (2005). Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. International Dairy Journal, 15(6–9), 711–718. https://doi.org/10.1016/j.idairyj.2004.09.012
Holmquist, M. (2000). Alpha beta-hydrolase fold enzymes structures, functions and mechanisms. Current Protein and Peptide Science, 1(2), 209–235. https://doi.org/10.2174/1389203003381405
Hongsawat, P., & Vangnai, A. S. (2011). Biodegradation pathways of chloroanilines by Acinetobacterbaylyi strain GFJ2. Journal of Hazardous Materials, 186(2–3), 1300–1307. https://doi.org/10.1016/j.jhazmat.2010.12.002
Hou, R., Hu, J., Wang, Y., Wei, H., & Gao, M. (2020). Simultaneous production of cellulase and ferulic acid esterase by Penicillium decumbens with rice straw as the sole carbon source. Journal of Bioscience and Bioengineering, 129(3), 276–283. https://doi.org/10.1016/j.jbiosc.2019.09.013
Huang, Z., Yu, K., Xiao, Y., Wang, Y., Xiao, D., & Wang, D. (2022). Comparative genomic analysis reveals potential pathogenicity and slow-growth characteristics of genus Brevundimonas and description of Brevundimonaspishanensis sp. nov. microbiology. Spectrum, 10(2), e02468-21. https://doi.org/10.1128/spectrum.02468-21
Jeong, Y. J., Baek, S. C., & Kim, H. (2018). Cloning and characterization of a novel intracellular serine protease (IspK) from Bacillus megaterium with a potential additive for detergents. International Journal of Biological Macromolecules, 108, 808–816. https://doi.org/10.1016/j.ijbiomac.2017.10.173
Jiang, Z., Qu, L., Song, G., Liu, J., & Zhong, G. (2022). The potential binding interaction and hydrolytic mechanism of carbaryl with the novel esterase PchA in Pseudomonas sp. PS21. Journal of Agricultural and Food Chemistry, 70(7), 2136–2145. https://doi.org/10.1021/acs.jafc.1c06465
Jo, E., Kim, J., Lee, A., Moon, K., & Cha, J. (2021). Identification and characterization of a novel thermostable GDSL-type lipase from Geobacillus thermocatenulatus. Journal of Microbiology and Biotechnology, 31(3), 483–491. https://doi.org/10.4014/jmb.2012.12036
Junghare, M., Spiteller, D., & Schink, B. (2019). Anaerobic degradation of xenobiotic isophthalate by the fermenting bacterium Syntrophorhabdusaromaticivorans. ISME Journal, 13(5), 1252–1268. https://doi.org/10.1038/s41396-019-0348-5
Kabir, S. M. M., & Koh, J. (2022). Sustainable textile processing by enzyme applications. Biodegradation Technology of Organic and Inorganic Pollutants. https://doi.org/10.5772/intechopen.97198
Kameda, T., Aoki, H., Yanaka, N., & Kato, N. (2018). Production of isoflavone aglycone-enriched tempeh with Rhizopus stolonifer. Journal of Food Science and Technology, 24(3), 493–499. https://doi.org/10.3136/fstr.24.493
Kameshwar, A. K. S., & Qin, W. (2018). Understanding the structural and functional properties of carbohydrate esterases with a special focus on hemicellulose deacetylating acetyl xylanesterases. The Journal of Mycology, 9(4), 273–295. https://doi.org/10.1080/21501203.2018.1492979
Keawmanee, P., Rattanakreetakul, C., & Pongpisutta, R. (2021). Microbial reduction of fumonisin B1 by the new isolate Serratia marcescens 329–2. Toxins, 13(9), 638. https://doi.org/10.3390/toxins13090638
Khan, N. R., & Rathod, V. K. (2015). Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochemistry, 50(11), 1793–1806. https://doi.org/10.1016/j.procbio.2015.07.014
Khan, Z., Javed, F., Shamair, Z., Hafeez, A., Fazal, T., Aslam, A., Zimmerman, W. B., & Rehman, F. (2021). Current developments in esterification reaction: A review on process and parameters. Journal of Industrial and Engineering Chemistry, 103, 80–101. https://doi.org/10.1016/j.jiec.2021.07.018
Kim, M., Jang, M., Nam, G., Shin, H., Song, J., & Kim, T. (2020). Functional expression and characterization of acetyl xylan esterases CE family 7 from Lactobacillusantri and Bacillushalodurans. Journal of Microbiology and Biotechnology, 30(2), 155–162. https://doi.org/10.4014/jmb.2001.01004
Kohli, P., & Gupta, R. (2016). Medical aspects of esterases: A mini review. International Journal of Pharmacy and Pharmaceutical Sciences, 8(8), 21–26.
Konuray, G., & Erginkaya, Z. (2018). Potential use of Bacillus coagulans in the food industry. Foods, 7(6), 92. https://doi.org/10.3390/foods7060092
Kour, D., Rana, K. L., Kaur, T., Singh, B., Chauhan, V., Kumar, A., Rastegari, A. A., Yadav, N., Yadav, A. N., & Gupta, V. K. (2019). Extremophiles for hydrolytic enzymes productions: Biodiversity and potential biotechnological applications (pp. 321–372). Wiley. https://doi.org/10.1002/9781119434436.ch16
Kovacic, F., Bleffert, F., Caliskan, M., Wilhelm, S., Granzin, J., Batra-Safferling, R., & Jaeger, K. (2016). A membrane-bound esterase PA2949 from Pseudomonas aeruginosais expressed and purified from Escherichia coli. FEBS Open Bio, 6(5), 484–493. https://doi.org/10.1002/2211-5463.12061
Larsen, E., & Johnson, R. J. (2018). Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance. Drug Development Research, 80(1), 33–47. https://doi.org/10.1002/ddr.21468
Lee, C. W., Kwon, S., Park, S., Kim, B., Yoo, W., Ryu, B. H., Kim, H., Shin, S. C., Kim, S., Park, H., Kim, T. D., & Lee, J. H. (2017). Crystal structure and functional characterization of an esterase (EaEST) from Exiguobacterium antarcticum. PLoS ONE, 12(1), e0169540. https://doi.org/10.1371/journal.pone.0169540
Li, L., Ding, L., Shao, Y., Sun, S., Wang, M., Xiang, J., Zhou, J., Wu, G., Song, Z., & Xin, Z. (2023). Enhancing the hydrolysis and acyl transfer activity of carboxylesterase DLFAE4 by a combinational mutagenesis and in-silico method. Foods, 12(6), 1169. https://doi.org/10.3390/foods12061169
Li, P., Chen, X., Ji, P., Li, C., Wang, P., Zhang, Y., Xie, B., Qin, Q., Su, H., Zhou, B., Zhang, Y., & Zhang, X. (2015). Interdomain hydrophobic interactions modulate the thermostability of microbial esterases from the hormone-sensitive lipase family. Journal of Biological Chemistry, 290(17), 11188–11198. https://doi.org/10.1074/jbc.m115.646182
Libik-Konieczny, M., Capecka, E., Tuleja, M., & Konieczny, R. (2021). Synthesis and production of steviol glycosides: Recent research trends and perspectives. Applied Microbiology and Biotechnology, 105(10), 3883–3900. https://doi.org/10.1007/s00253-021-11306-x
López-López, O., Cerdán, M. E., & Siso, M. (2014). New extremophilic lipases and esterases from metagenomics. Current Protein and Peptide Science, 15(5), 445–455. https://doi.org/10.2174/1389203715666140228153801
Lu, M., Tian, X., Tian, A., Li, C., Ren, Y., Xu, L., Song, X., & Li, X. (2020). A novel α/β Hydrolase domain protein derived from Haemonchuscontortus acts at the parasite-host interface. Frontiers Immunology, 11, 1388. https://doi.org/10.3389/fimmu.2020.01388
Lundberg, D., Stjerndahl, M., & Holmberg, K. (2022). Ester-based surfactants: Are they stable enough? Journal of Surfactants and Detergents, 26(3), 229–236. https://doi.org/10.1002/jsde.12628
Maestri, C., Plancher, L., Duthoit, A., Hébert, R., & Di Martino, P. (2023). Fungal biodegradation of polyurethanes. Journal of Fungus, 9(7), 760. https://doi.org/10.3390/jof9070760
Mali, H., Shah, C., Patel, D. H., Trivedi, U., & Subramanian, R. B. (2022). Degradation insight of organophosphate pesticide chlorpyrifos through novel intermediate 2, 6-dihydroxypyridine by Arthrobacter sp. HM01. Bioresources and Bioprocessing, 9(1), 1–14. https://doi.org/10.1186/s40643-022-00515-5
Marchot, P., & Chatonnet, A. (2012). Enzymatic activity and protein interactions in alpha/beta hydrolase fold proteins: Moonlighting versus promiscuity. Protein and Peptide Letters, 19(2), 132–143. https://doi.org/10.2174/092986612799080284
Mate, D. M., Rivera, N. R., Sanchez-Freire, E., Ayala, J. A., Berenguer, J., & Hidalgo, A. (2019). Thermostability enhancement of the Pseudomonas fluorescens esterase I by in vivo folding selection in Thermus thermophilus. Biotechnology and Bioengineering, 117(1), 30–38. https://doi.org/10.1002/bit.27170
Matrawy, A. A., Khalil, A., & Embaby, A. M. (2022). Molecular study on recombinant cold-adapted, detergent- and alkali stable esterase (EstRag) from Lysinibacillus sp.: A member of family VI. World Journal of Microbiology and Biotechnology, 38(12), 217. https://doi.org/10.1007/s11274-022-03402-5
Mesbah, N. M. (2022). Industrial biotechnology based on enzymes from extreme environments. Frontiers in Bioengineering and Biotechnology, 10, 870083. https://doi.org/10.3389/fbioe.2022.870083
Mostafa, A. A., Al-Askar, A. A., Almaary, K. S., Dawoud, T. M., Sholkamy, E. N., & Bakri, M. M. (2018). Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi Journal of Biological Sciences, 25(2), 361–366. https://doi.org/10.1016/j.sjbs.2017.02.004
Mukdsi, M. A., Falentin, H., Maillard, M., Chuat, V., Medina, R., Parayre, S., & Thierry, A. (2014). The secreted esterase of Propionibacteriumfreudenreichii has a major role in cheese lipolysis. Applied and Environment Microbiology, 80(2), 751–756. https://doi.org/10.1128/aem.03640-13
Nehra, V., Saharan, B. S., & Choudhary, M. (2016). Evaluation of Brevibacillus brevis as a potential plant growth promoting rhizobacteria for cotton (Gossypium hirsutum) crop. Springerplus, 5, 1–10. https://doi.org/10.1186/s40064-016-2584-8
Ng, A. M. J., Zhang, H., & Nguyen, G. K. T. (2021). Zymography for picogram detection of lipase and esterase activities. Molecules, 26(6), 1542. https://doi.org/10.3390/molecules26061542
Niyonzima, F. N., & More, S. S. (2014). Purification and characterization of detergent-compatible protease from Aspergillusterreus gr. 3 Biotech, 5(1), 61–70. https://doi.org/10.1007/s13205-014-0200-6
Noor, H., Satti, S. M., Din, S. U., Farman, M., Hasan, F., Khan, S., Badshah, M., & Shah, A. A. (2020). Insight on esterase from Pseudomonas aeruginosa strain S3 that depolymerize poly(lactic acid) (PLA) at ambient temperature. Polymer Degradation and Stability, 174, 109096. https://doi.org/10.1016/j.polymdegradstab.2020.109096
Nutschel, C., Coscolín, C., David, B., Mulnaes, D., Ferrer, M., Jaeger, K., & Gohlke, H. (2021). Promiscuous esterases counterintuitively are less flexible than specific ones. Journal of Chemical Information and Modeling, 61(5), 2383–2395. https://doi.org/10.1021/acs.jcim.1c00152
Nyanhongo, G. S., Acero, E. H., Matuchaki, M. D. D. J., Rau, M., Guebitz, G. M., Andreaus, J., Gupta, V. K., Zeilinger, S., Filho, E. X. F., Durán-Dominguez-De-Bazu, M. C., Purchase, D. (2016) 2. Microbial applications for fabric and textile industries. In De Gruyter eBooks (pp. 33–78). https://doi.org/10.1515/9783110412789-004
Oh, C. W., Kim, T. D., & Kim, K. K. (2019). Carboxylic ester hydrolases in bacteria: Active site, structure, function and application. Crystals, 9(11), 597. https://doi.org/10.3390/cryst9110597
Öz, Y., Sürmeli, Y., & Şanlı-Mohamed, G. (2022). Enhanced thermostability of the immobilized thermoalkalophilic esterase onto magnetic-cornstarch nanoparticle. Biotechnology and Applied Biochemistry, 69(4), 1418–1427. https://doi.org/10.1002/bab.2213
Panda, T., & Gowrishankar, B. S. (2005). Production and applications of esterases. Applied Microbiology and Biotechnology, 67(2), 160–169. https://doi.org/10.1007/s00253-004-1840-y
Park, J., Jeong, G., Lee, H., & Kim, H. (2021). Molecular characterization of novel family IV and VIII esterases from a compost metagenomic library. Microorganisms, 9(8), 1614. https://doi.org/10.3390/microorganisms9081614
Pérez-Rodríguez, N., Moreira, C., Agrasar, A. T., & Domínguez, J. M. (2016). Feruloyl esterase production by Aspergillus terreus CECT 2808 and subsequent application to enzymatic hydrolysis. Enyzme and Microbial Technology, 91, 52–58. https://doi.org/10.1016/j.enzmictec.2016.05.011
Qiao, J., Yang, D., Feng, Y., Wan, W., Liu, X., Zhang, Y., Zheng, J., & Ying, X. (2023). Engineering a Bacillus subtilis esterase for selective hydrolysis of d, l-menthyl acetate in an organic solvent-free system. RSC Advances, 13(16), 10468–10475. https://doi.org/10.1039/d3ra00490b
Ramnath, L., Sitholé, B., & Govinden, R. (2017). Identification of lipolytic enzymes isolated from bacteria indigenous to eucalyptus wood species for application in the pulping industry. Biotechnology Reports, 15, 114–124. https://doi.org/10.1016/j.btre.2017.07.004
Rauwerdink, A., & Kazlauskas, R. J. (2015). How the same core catalytic machinery catalyzes 17 different reactions: The serine-histidine-aspartate catalytic triad of α/β-hydrolase fold enzymes. ACS Catalysis, 5(10), 6153–6176. https://doi.org/10.1021/acscatal.5b01539
Romero-Rivera, A., Garcia-Borràs, M., & Osuna, S. (2017). Computational tools for the evaluation of laboratory-engineered biocatalysts. Chemical Communications, 53(2), 284–297. https://doi.org/10.1039/c6cc06055b
Rose, R., Richardson, K. H., Latvanen, E. J., Hanson, C. A., Resmini, M., & Sanders, I. A. (2020). Microbial degradation of plastic in aqueous solutions demonstrated by CO2 evolution and quantification. International Journal of Molecular Sciences, 21(4), 1176. https://doi.org/10.3390/ijms21041176
Saghatelyan, A., Margaryan, A., Panosyan, H., & Birkeland, N. K. (2021). Microbial diversity of terrestrial geothermal springs in Armenia and Nagorno-Karabakh: A review. Microorganisms, 9(7), 1473. https://doi.org/10.3390/microorganisms9071473
Saitta, F., Cannazza, P., Donzella, S., De Vitis, V., Signorelli, M., Romano, D., Molinari, F., & Fessas, D. (2022). Calorimetric and thermodynamic analysis of an enantioselective carboxylesterase from Bacillus coagulans: Insights for an industrial scale-up. Thermochimica Acta, 713, 179247. https://doi.org/10.1016/j.tca.2022.179247
Sajid, M. A. (2022). Transesterification: Mechanism and Applications. PSIBERG. https://psiberg.com/transesterification/
Sales, J. C. S., De Castro, A. M., Ribeiro, B. D., & Coelho, M. A. Z. (2020). Supplementation of watermelon peels as an enhancer of lipase and esterase production byYarrowia lipolyticain solid-state fermentation and their potential use as biocatalysts in poly(ethylene terephthalate) (PET) depolymerization reactions. Journal of Biocatalysis and Biotransformation, 38(6), 457–468. https://doi.org/10.1080/10242422.2020.1782387
Sanchez-Hernandez, J. C. (2010). Environmental applications of earthworm esterases in the agroecosystem. Journal of Pesticide Science, 35(3), 290–301. https://doi.org/10.1584/jpestics.r10-08
Sarnaik, A., Shinde, S., Mhatre, A., Jansen, A., Jha, A., Mckeown, H., Davis, R. W., & Varman, A. M. (2023). Unravelling the hidden power of esterases for biomanufacturing of short-chain esters. Scientific Report, 13(1), 10766. https://doi.org/10.1038/s41598-023-37542-x
Sayali, K., Sadichha, P., & Surekha, S. (2013). Microbial esterases: An overview. International Journal of Current Microbiology Applied Science, 2(7), 135–146.
Sayer, C., Szabó, Z., Isupov, M. N., Ingham, C. J., & Littlechild, J. A. (2015). The structure of a novel thermophilic esterase from the planctomycetes species, thermoguttaterrifontisreveals an open active site due to a minimal ‘cap’ domain. Frontiers Microbiology, 6, 01294. https://doi.org/10.3389/fmicb.2015.01294
Shang, N., Chen, L., Cheng, M., Tian, Y., & Huang, X. (2022). Biodegradation of diphenyl ether herbicide lactofen by Bacillus sp. YS-1 and characterization of two initial degrading esterases. Science of the Total Environment, 806, 151357. https://doi.org/10.1016/j.scitotenv.2021.151357
Sharma, T., Sharma, A., & Kanwar, S. S. (2017). An overview on esterases: Structure, classification, sources and their application. Recent Advances in Biotechnology, 2, 216–229.
Shi, K., Jing, J., Li, S., Su, T., & Wang, Z. (2020). Enzymatic hydrolysis of polyester: Degradation of poly (ε-caprolactone) by Candida antarctica lipase and Fusarium solani cutinase. International Journal of Biological Macromolecules, 144, 183–189. https://doi.org/10.1016/j.ijbiomac.2019.12.105
Silva, C., Azoia, N. G., Martins, M., Matamá, T., Wu, J., & Cavaco-Paulo, A. (2012). Molecular recognition of esterase plays a major role on the removal of fatty soils during detergency. Journal of Biotechnology, 161(3), 228–234. https://doi.org/10.1016/j.jbiotec.2012.06.019
Singh, L., Sharma, G., Awasthi, G., Kumar, L., Ali, M. I., & Moin, S. (2019). Screening, isolation and identification of thermophilic esterase enzyme isolated from Rhodococcus sp. LKE-021. Journal Pure and Applied Microbiology, 13(3), 1855–1861.
Sree, V. G., Archana, D., Prithika, U., Sivapriya, E., Tejaswi, B., & PradeepKumar, A. R. (2020). Esterase like activity of Enterococcus faecalis and Lactobacillus casei on microhardness and weight loss of resin luting cements. Indian Journal of Dental Research. https://doi.org/10.4103/ijdr.ijdr_747_20
Sun, X., Griffith, M., Pasternak, J., & Glick, B. R. (1995). Low temperature growth, freezing survival, and production of antifreeze protein by the plant growth promoting rhizobacteriumPseudomonas putidaGR12-2. Canadian Journal of Microbiology, 41(9), 776–784. https://doi.org/10.1139/m95-107
Szczęsna-Antczak, M., Struszczyk-Świta, K., Rzyska, M., Szeląg, J., Stańczyk, Ł, & Antczak, T. (2018). Oil accumulation and in situ trans/esterification by lipolytic fungal biomass. Bioresource Technology, 265, 110–118. https://doi.org/10.1016/j.biortech.2018.05.094
Szczyrba, E., Greń, I., & Bartelmus, G. (2013). Enzymes involved in vinyl acetate decomposition by Pseudomonas fluorescens PCM 2123 strain. Folia Microbiologica, 59(2), 99–105. https://doi.org/10.1007/s12223-013-0268-0
Tamoor, M., Samak, N. A., Jia, Y., Mushtaq, M. U., Sher, H., Bibi, M., & Xing, J. (2021). Potential use of microbial enzymes for the conversion of plastic waste into value-added products: A viable solution. Frontiers Microbiology, 12, 777727. https://doi.org/10.3389/fmicb.2021.777727
Timkina, E., Drabova, L., Palyzová, A., Řezanka, T., Maťátková, O., & Kolouchová, I. (2022). Kocuria strains from unique radon spring water from Jachymov Spa. Journal of Fermentation, 8(1), 35. https://doi.org/10.3390/fermentation8010035
Ufarté, L., Laville, E., Duquesne, S., & Potocki-Veronese, G. (2015). Metagenomics for the discovery of pollutant degrading enzymes. Biotechnology Advances, 33(8), 1845–1854. https://doi.org/10.1016/j.biotechadv.2015.10.009
Underlin, E. N., Frommhagen, M., Dilokpimol, A., Van Erven, G., & Kabel, M. A. (2020). Feruloyl esterases for biorefineries: Subfamily classified specificity for natural substrates. Frontiers Bioengineering Biotechnology, 8, 332. https://doi.org/10.3389/fbioe.2020.00332
Vaquero, M. E., Barriuso, J., Medrano, F., Prieto, A., & Martínez, M. J. (2015). Heterologous expression of a fungal sterol esterase/lipase in different hosts: Effect on solubility, glycosylation and production. Journal of Bioscience and Bioengineering, 120(6), 637–643. https://doi.org/10.1016/j.jbiosc.2015.04.005
Virk, A. P., Puri, M., Gupta, V., Capalash, N., & Sharma, P. (2013). Combined enzymatic and physical deinking methodology for efficient eco-friendly recycling of old newsprint. PLoS ONE, 8(8), e72346. https://doi.org/10.1371/journal.pone.0072346
Wang, H., & Wang, J. (2016). How cryo-electron microscopy and X-ray crystallography complement each other. Protein Science, 26(1), 32–39. https://doi.org/10.1002/pro.3022
Wang, Y., Xu, Y., Zhang, Y., Sun, A., & Hu, Y. (2018). Functional characterization of salt-tolerant microbial esterase WDEst17 and its use in the generation of optically pure ethyl (R)-3-hydroxybutyrate. Chirality, 30(6), 769–776. https://doi.org/10.1002/chir.22847
Wang, Y., Yang, C., Huang, K., & Shaw, J. (2021). Chlorophyllides: Preparation, purification, and application. Biomolecules, 11(8), 1115. https://doi.org/10.3390/biom11081115
Wu, H., Yang, Y., Wang, S., Qiao, J., Xia, Y., Wang, Y., Wang, W., Gao, S., Liu, J., Xue, P., & Gao, X. (2011). Cloning, expression and characterization of a new aspartate aminotransferase from Bacillus subtilis B3. FEBS Journal, 278(8), 1345–1357. https://doi.org/10.1111/j.1742-4658.2011.08054.x
Xu, A., Zhang, X., Wu, S., Xu, N., Huang, Y., Yan, X., Zhou, J., Cui, Z., & Dong, W. (2021). Pollutant degrading enzyme: Catalytic mechanisms and their expanded applications. Molecules, 26(16), 4751. https://doi.org/10.3390/molecules26164751
Xue, D., Yao, D., You, X., & Gong, C. (2020). Green synthesis of the flavor esters with a marine Candida parapsilosis esterase expressed in Saccharomyces cerevisiae. Indian Journal of Microbiology, 60(2), 175–181. https://doi.org/10.1007/s12088-020-00856-9
Yang, Y., Yang, G., Hu, J., & Wang, M. (2018). Effect of mono-, di-, and trihydric alcohols on lipase-catalyzed alcoholysis of phosphatidylcholine in hexane. Grain and Oil Science and Technology, 1(2), 105–108. https://doi.org/10.3724/sp.j.1447.gost.2018.18030
Yi, Y., Yang, J., Li, B., Wang, E. T., & Yuan, H. (2018). An esterase from Penicillium decumbens P6 involved in lignite depolymerization. Fuel, 214, 416–422. https://doi.org/10.1016/j.fuel.2017.11.035
Zarafeta, D., Szabó, Z., Moschidi, D., Phan, H., Chrysina, E. D., Peng, X., Ingham, C. J., Kolisis, F. N., & Skretas, G. (2016). ESTDZ3: A new esterolytic enzyme exhibiting remarkable thermostability. Frontiers Microbiology, 7, 1779. https://doi.org/10.3389/fmicb.2016.01779
Zeng, H., Wang, Y., Han, H., Cao, Y., & Wang, B. (2022). Changes in key aroma compounds and esterase activity of monascus-fermented cheese across a 30-day ripening period. Foods, 11(24), 4026. https://doi.org/10.3390/foods11244026
Zeng, S., Liu, J., Anankanbil, S., Chen, M., Guo, Z., Adams, J. P., Snajdrova, R., & Li, Z. (2018). Amide synthesis via aminolysis of ester or acid with an intracellular lipase. ACS Catalysis, 8(9), 8856–8865. https://doi.org/10.1021/acscatal.8b02713
Zhang, H., Xia, Y., Zhou, M., Zheng, J., Wang, Z., & Zhang, Y. (2019). Purification and characterization of a thermoalkaliphilic esterase from Bacillus cereus WZZ006 for enantioselective resolution of indoxacarb intermediate. International Journal of Biological Macromolecules, 140, 358–367. https://doi.org/10.1016/j.ijbiomac.2019.08.140
Acknowledgements
This work is carried out with the help of prestigious material from the libraries and special thanks to the Institutes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The authors declare that they have no competing financial interests. We assure the integrity and quality of our research work. It is also stated that there is no plagiarism in this work and all points taken from other authors are well cited in the text. This study is completely independent and impartial.
Human and Animals Participants
N/A. This research did not involve human participants and/or animals.
Informed Consent
N/A. This research did not involve human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akram, F., Fatima, T., Shabbir, I. et al. Abridgement of Microbial Esterases and Their Eminent Industrial Endeavors. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01108-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01108-7