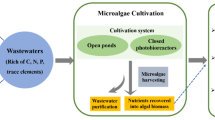
Abstract
Microalgal species from sewage treatment plant were identified by 18S rRNA sequencing and were explored for total lipids, carbohydrate, and protein contents, to serve as a potential candidate for biorefinery. Seven unicellular microalgae were identified as Chlorella sorokiniana, Dictyosphaerium sp., Graesiella emersonii belonging to Chlorellaceae and Scenedesmus sp., Desmodesmus sp., Tetranephris brasiliensis, and Coelastrella sp. belonging to Scenedesmaceae family. Biochemical assessment of all isolates revealed total lipid content from 17.49 ± 1.41 to 47.35 ± 0.61% w/w, total carbohydrate content from 12.82 ± 0.19 to 64.29 ± 0.63% w/w, and total protein content from 8.55 ± 0.19 to 16.65 ± 0.20% w/w. FAME analysis of extracted lipid was found to be rich in Hexadecane (C16:0), Tetradecane (C17:0), Octadecane (C18:0), Eicosane (C20:0), Tetracosane (C24:0), Pentacosane (C25:0) fatty acids, the presence of which makes excellent candidate for biodiesel. Being rich in lipid, microalgae Chlorella sorokiniana, Coelastrella sp., and Scenedesmus sp. have high potential for biofuels. Due to the presence of high protein content, Scenedesmus sp. and Chlorella sorokiniana can serve as food or feed supplement, whereas the high carbohydrate content of Dictyosphaerium sp., Coelastrella sp., and Scenedesmus sp. makes them an ideal candidate for fermentative production of alcohol and organic acids. Chlorella sp. and Scenedesmus sp., being dominant microalgae across all seasons, demonstrate remarkable resilience for their cultivation in sewage water and utilization of biomass in biorefineries.







Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Massimi, R., & Kirkwood, A. E. (2016). Screening microalgae isolated from urban storm and wastewater systems as feedstock for biofuel. PeerJ, 4, 2396. https://doi.org/10.7717/peerj.2396
Sahoo, D., Elangbam, G., & Devi, S. S. (2012). Using algae for carbon dioxide capture and biofuel production to combat climate change. Phykos, 42(1), 32–38.
Kanth, B. K., Min, K., Kumari, S., Jeon, H., Jin, E. S., Lee, J., & Pack, S. P. (2012). Expression and characterization of codon-optimized carbonic anhydrase from Dunaliella species for CO\(_2\) sequestration application. Applied Biochemistry and Biotechnology, 167(8), 2341–2356. https://doi.org/10.1007/s12010-012-9729-1
Kim, J. K., Um, B.-H., & Kim, T. H. (2011). Bioethanol production from micro-algae, Schizocytrium sp., using hydrothermal treatment and biological conversion. Korean Journal of Chemical Engineering, 29(2), 209–214. https://doi.org/10.1007/s11814-011-0169-3
Wang, L., Min, M., Li, Y., Chen, P., Chen, Y., Liu, Y., Wang, Y., & Ruan, R. (2009). Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Applied Biochemistry and Biotechnology, 162(4), 1174–1186. https://doi.org/10.1007/s12010-009-8866-7
Capron, M. E., Stewart, J. R., de Ramon N’Yeurt, A., Chambers, M. D., Kim, J. K., Yarish, C., Jones, A. T., Blaylock, R. B., James, S. C., Fuhrman, R., Sherman, M. T., Piper, D., Harris, G., & Hasan, M. A. (2020). Restoring pre-industrial CO2 levels while achieving sustainable development goals. Energies, 13(18), 4972. https://doi.org/10.3390/en13184972
Zhou, X., Jin, W., Wang, Q., Guo, S., Tu, R., Han, S.-F., Chen, C., Xie, G., Qu, F., & Wang, Q. (2020). Enhancement of productivity of Chlorella pyrenoidosa lipids for biodiesel using co-culture with ammonia-oxidizing bacteria in municipal wastewater. Renewable Energy, 151, 598–603. https://doi.org/10.1016/j.renene.2019.11.063
Choudhary, P., Prajapati, S. K., & Malik, A. (2016). Screening native microalgal consortia for biomass production and nutrient removal from rural wastewaters for bioenergy applications. Ecological Engineering, 91, 221–230. https://doi.org/10.1016/j.ecoleng.2015.11.056
Kim, E. J., Kim, S., Choi, H.-G., & Han, S. J. (2020). Co-production of biodiesel and bioethanol using psychrophilic microalga Chlamydomonas sp. KNM0029C isolated from arctic sea ice. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-020-1660-z
Mondal, P., & Dalai, A. K. (2017). Sustainable utilization of natural resources. CRC Press. https://doi.org/10.1201/9781315153292
Nawkarkar, P., Singh, A. K., Abdin, M. Z., & Kumar, S. (2019). Life cycle assessment of Chlorella species producing biodiesel and remediating wastewater. Journal of Biosciences. https://doi.org/10.1007/s12038-019-9896-0
Bi, Z., & He, B. B. (2013). Characterization of microalgae for the purpose of biofuel production. American Society of Agricultural and Biological Engineers, 56(4), 1529–1539. https://doi.org/10.13031/trans.56.10090
Kim, B., Kim, W., & Choi, H. (2010). Seasonal variability of seaweed biomass along the vertical shore gradients of Nachido and Odo islands, the Yellow Sea. Korea. Fish Aqua Sci, 13, 324–331. https://doi.org/10.5657/fas.2010.13.4.324
Kaur, H., & Kaur, P. (2015). Temperature features in different agroclimatic zones of Punjab. Agricultural Research Journal, 52(4), 32. https://doi.org/10.5958/2395-146x.2015.00057.5
Kang, J.-C., Choi, H.-G., & Kim, M.-S. (2011). Macroalgal species composition and seasonal variation in biomass on Udo, Jeju island, Korea. ALGAE, 26(4), 333–342. https://doi.org/10.4490/algae.2011.26.4.333
Zhou, W., Li, Y., Min, M., Hu, B., Chen, P., & Ruan, R. (2011). Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresource Technology, 102(13), 6909–6919. https://doi.org/10.1016/j.biortech.2011.04.038
Caporgno, M. P., Taleb, A., Olkiewicz, M., Font, J., Pruvost, J., Legrand, J., & Bengoa, C. (2015). Microalgae cultivation in urban wastewater: Nutrient removal and biomass production for biodiesel and methane. Algal Research, 10, 232–239. https://doi.org/10.1016/j.algal.2015.05.011
Han, S.-F., Jin, W., Abomohra, A.E.-F., Zhou, X., Tu, R., Chen, C., Chen, H., Gao, S.-H., & Wang, Q. (2019). Enhancement of lipid production of Scenedesmus obliquus cultivated in municipal wastewater by plant growth regulator treatment. Waste and Biomass Valorization, 10(9), 2479–2485.
Lee, C. S., Oh, H.-S., Oh, H.-M., Kim, H.-S., & Ahn, C.-Y. (2016). Two-phase photoperiodic cultivation of algal–bacterial consortia for high biomass production and efficient nutrient removal from municipal wastewater. Bioresource Technology, 200, 867–875. https://doi.org/10.1016/j.biortech.2015.11.007
Stemmler, K., Massimi, R., & Kirkwood, A. E. (2016). Growth and fatty acid characterization of microalgae isolated from municipal waste-treatment systems and the potential role of algal-associated bacteria in feedstock production. PeerJ, 4, 1780. https://doi.org/10.7717/peerj.1780
Singh, A. K., Sharma, N., Farooqi, H., Abdin, M. Z., Mock, T., & Kumar, S. (2017). Phycoremediation of municipal wastewater by microalgae to produce biofuel. International Journal of Phytoremediation, 19(9), 805–812. https://doi.org/10.1080/15226514.2017.1284758
Alam, M. A., & Wang, Z. (2019). Microalgae biotechnology for development of biofuel and wastewater treatment. Springer Singapore. https://doi.org/10.1007/978-981-13-2264-8
Makut, B. B., Das, D., & Goswami, G. (2019). Production of microbial biomass feedstock via co-cultivation of microalgae–bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Research, 37, 228–239. https://doi.org/10.1016/j.algal.2018.11.020
Baldisserotto, C., Demaria, S., Arcidiacono, M., Benà, E., Giacò, P., Marchesini, R., Ferroni, L., Benetti, L., Zanella, M., Benini, A., & Pancaldi, S. (2023). Enhancing urban wastewater treatment through isolated Chlorella strain-based phytoremediation in centrate stream: An analysis of algae morpho-physiology and nutrients removal efficiency. Plants, 12(5), 1027. https://doi.org/10.3390/plants12051027
Eladel, H., Abomohra, A.E.-F., Battah, M., Mohmmed, S., Radwan, A., & Abdelrahim, H. (2018). Evaluation of Chlorella sorokiniana isolated from local municipal wastewater for dual application in nutrient removal and biodiesel production. Bioprocess and Biosystems Engineering, 42(3), 425–433. https://doi.org/10.1007/s00449-018-2046-5
Pandey, A., Srivastava, S., & Kumar, S. (2019). Isolation, screening and comprehensive characterization of candidate microalgae for biofuel feedstock production and dairy effluent treatment: A sustainable approach. Bioresource Technology, 293, 121998. https://doi.org/10.1016/j.biortech.2019.121998
Kamyab, Hesam, Din, M. F. M., Ponraj, M., Keyvanfar, A., Rezania, S., Taib, S. M., & Majid, M. Z. A. (2016). Isolation and screening of microalgae from agro-industrial wastewater (POME) for biomass and biodiesel sources. Desalination and Water Treatment, 57(60), 29118–29125. https://doi.org/10.1080/19443994.2016.1139101
Yang, Y., Tang, F., Su, X., Yin, H., & Ge, F. (2016). Identification and evaluation of a dominant alga from municipal wastewater in removal of nutrients. Water Science and Technology, 74(11), 2727–2735. https://doi.org/10.2166/wst.2016.437
Allen, M. M., & Stanier, R. Y. (1968). Growth and division of some unicellular blue-green algae. Journal of General Microbiology, 51(2), 199–202. https://doi.org/10.1099/00221287-51-2-199
Renuka, N., Sood, A., Ratha, S. K., Prasanna, R., & Ahluwalia, A. S. (2013). Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. Journal of Applied Phycology, 25(5), 1529–1537. https://doi.org/10.1007/s10811-013-9982-x
Whitton, R., Ometto, F., Villa, R., Pidou, M., & Jefferson, B. (2019). Influence of light regime on the performance of an immobilised microalgae reactor for wastewater nutrient removal. Algal Research, 44, 101648. https://doi.org/10.1016/j.algal.2019.101648
Andersen, R. A. (2005). Algal culturing techniques. Elsevier.
Desikachary, T. (1959). Cyanophyta, monograph on blue green algae (pp. 1–689). ICAR.
Rippka, R., Stanier, R. Y., Deruelles, J., Herdman, M., & Waterbury, J. B. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology, 111(1), 1–61. https://doi.org/10.1099/00221287-111-1-1
Guiry, M. D., Guiry, G. M., Morrison, L., Rindi, F., Miranda, S. V., Mathieson, A. C., Parker, B. C., Langangen, A., John, D. M., & Bárbara, I. (2014). Algaebase: an on-line resource for algae. Cryptogamie, Algologie, 35(2), 105–115.
Karlin, S., & Altschul, S. F. (1990). Methods for assessing the statistical significance of molecular sequence features by using general scoring schemes. Proceedings of the National Academy of Sciences, 87(6), 2264–2268. https://doi.org/10.1073/pnas.87.6.2264
Myers, N. (1990). The biodiversity challenge: Expanded hot-spots analysis. The Environmentalist, 10(4), 243–256. https://doi.org/10.1007/bf02239720
Surzycki, S. (2000). Preparation of genomic DNA from bacteria. Basic Techniques in Molecular Biology. https://doi.org/10.1007/978-3-642-56968-5_4
Darby, A. C., Chandler, S. M., Welburn, S. C., & Douglas, A. E. (2005). Aphid-symbiotic bacteria cultured in insect cell lines. Applied and Environmental Microbiology, 71(8), 4833–4839. https://doi.org/10.1128/aem.71.8.4833-4839.2005
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols. https://doi.org/10.1016/b978-0-12-372180-8.50042-1
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410. https://doi.org/10.1016/s0022-2836(05)80360-2
Gertz, E. M., Yu, Y.-K., Agarwala, R., Schäffer, A. A., & Altschul, S. F. (2006). Composition-based statistics and translated nucleotide searches: Improving the tblastn module of blast. BMC Biology. https://doi.org/10.1186/1741-7007-4-41
Zhang, Z., Schwartz, S., Wagner, L., & Miller, W. (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology, 7(1–2), 203–214. https://doi.org/10.1089/10665270050081478
States, D., Gish, W., & Altschul, S. (1991). Improved sensitivity of nucleic acid database searches using application-specific scoring matrices. Methods, 3(1), 66–70. https://doi.org/10.1016/s1046-2023(05)80165-3
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917. https://doi.org/10.1139/o59-099
Bagchi, S. K., Rao, P. S., & Mallick, N. (2015). Development of an oven drying protocol to improve biodiesel production for an indigenous chlorophycean microalga Scenedesmus sp. Bioresource Technology, 180, 207–213. https://doi.org/10.1016/j.biortech.2014.12.092
Ogbonna, I. O., & Ogbonna, J. C. (2018). Effects of carbon source on growth characteristics and lipid accumulation by microalga Dictyosphaerium sp. with potential for biodiesel production. Energy and Power Engineering, 10(02), 29–42. https://doi.org/10.4236/epe.2018.102003
Pratoomyot, J., Srivilas, P., & Noiraksar, T. (2005). Fatty acids composition of 10 microalgal species. Songklanakarin Journal of Science and Technology, 27(6), 1179–1187.
McCurry, J. (2012). GC analysis of total fatty acid methyl esters (FAME) and methyl linolenate in biodiesel using the revised EN14103: 2011 method. Agilent Technologies Inc.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
Lowry, O., Rosebrough, N., Farr, A. L., & Randall, R. (1951). Protein measurement with the Folin-phenol reagent. Journal of Biological Chemistry, 193(1), 265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Segal, L., Creely, J. J., Martin, A. E., & Conrad, C. M. (1959). An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Textile Research Journal, 29(10), 786–794. https://doi.org/10.1177/004051755902901003
Ferro, L., Gojkovic, Z., Gorzsás, A., & Funk, C. (2019). Statistical methods for rapid quantification of proteins, lipids, and carbohydrates in nordic microalgal species using ATR–FTIR spectroscopy. Molecules, 24(18), 3237. https://doi.org/10.3390/molecules24183237
Meng, Y., Yao, C., Xue, S., & Yang, H. (2014). Application of Fourier Transform Infrared (FT-IR) spectroscopy in determination of microalgal compositions. Bioresource Technology, 151, 347–354. https://doi.org/10.1016/j.biortech.2013.10.064
Pancha, I., Chokshi, K., Maurya, R., Bhattacharya, S., Bachani, P., & Mishra, S. (2016). Comparative evaluation of chemical and enzymatic saccharification of mixotrophically grown de-oiled microalgal biomass for reducing sugar production. Bioresource Technology, 204, 9–16. https://doi.org/10.1016/j.biortech.2015.12.078
Arif, M., Li, Y., El-Dalatony, M. M., Zhang, C., Li, X., & Salama, E.-S. (2021). A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: An approach for biofuel generation and nutrients removal. Renewable Energy, 163, 1973–1982. https://doi.org/10.1016/j.renene.2020.10.066
Ferreira, G. F., Pinto, L. F. R., Carvalho, P. O., Coelho, M. B., Eberlin, M. N., Filho, R. M., & Fregolente, L. V. (2019). Biomass and lipid characterization of microalgae genera Botryococcus, Chlorella, and Desmodesmus aiming high-value fatty acid production. Biomass Conversion and Biorefinery, 11(5), 1675–1689. https://doi.org/10.1007/s13399-019-00566-3
World Health Organization. (2008). Interim summary of conclusions and dietary recommendations on total fat and fatty acids. From the joint FAO/WHO expert consultation on fats and fatty acids in human nutrition (pp. 10–14). WHO.
Grover, S., Kumari, P., Kumar, A., Soni, A., Sehgal, S., & Sharma, V. (2020). Preparation and quality evaluation of different oil blends. Letters in Applied NanoBioScience, 10(2), 2126–2137. https://doi.org/10.33263/lianbs102.21262137
Lee, J.-C., Joo, J.-H., Chun, B. H., Moon, K., Song, S. H., Kim, Y. J., Lee, S. M., & Lee, A. H. (2022). Isolation and screening of indigenous microalgae species for domestic and livestock wastewater treatment, biodiesel production, and carbon sequestration. Journal of Environmental Management, 318, 115648. https://doi.org/10.1016/j.jenvman.2022.115648
Fernández-Linares, L. C., Barajas, C. G., Páramo, E. D., & Corona, J. A. B. (2017). Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresource Technology, 244, 400–406. https://doi.org/10.1016/j.biortech.2017.07.141
Leong, W. H., Saman, N. A. M., Kiatkittipong, W., Assabumrungrat, S., Najdanovic-Visak, V., Wang, J., Khoo, K. S., Lam, M. K., Mohamad, M., & Lim, J. W. (2022). Photoperiod-induced mixotrophic metabolism in Chlorella vulgaris for high biomass and lipid to biodiesel productions using municipal wastewater medium. Fuel, 313, 123052. https://doi.org/10.1016/j.fuel.2021.123052
Samadhiya, K., Ghosh, A., Kashyap, M., Anand, V., & Bala, K. (2021). Bioprospecting of native algal strains with unique lipids, proteins, and carbohydrates signatures: A time dependent study. Environmental Progress Sustainable Energy. https://doi.org/10.1002/ep.13735
Kang, Nam Seon, Cho, K., An, S. M., Kim, E. S., Ki, H., Lee, C. H., Choi, G., & Hong, J. W. (2022). Taxonomic and biochemical characterization of microalga Graesiella emersonii GEGS21 for its potential to become feedstock for biofuels and bioproducts. Energies, 15(22), 8725. https://doi.org/10.3390/en15228725
Zhang, S., Liu, P.-H., Yang, X., Hao, Z.-D., Zhang, L., Luo, N., & Shi, J. (2014). Isolation and identification by 18s rDNA sequence of high lipid potential microalgal species for fuel production in Hainan Dao. Biomass and Bioenergy, 66, 197–203. https://doi.org/10.1016/j.biombioe.2014.01.015
Gour, R. S., Chawla, A., Singh, H., Chauhan, R. S., & Kant, A. (2016). Characterization and screening of native Scenedesmus sp. isolates suitable for biofuel feedstock. PLoS ONE, 11(5), 0155321. https://doi.org/10.1371/journal.pone.0155321
Kobayashi, N., Noel, E. A., Barnes, A., Watson, A., Rosenberg, J. N., Erickson, G., & Oyler, G. A. (2013). Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresource Technology, 150, 377–386. https://doi.org/10.1016/j.biortech.2013.10.032
Wan, M.-X., Wang, R.-M., Xia, J.-L., Rosenberg, J. N., Nie, Z.-Y., Kobayashi, N., Oyler, G. A., & Betenbaugh, M. J. (2012). Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnology and Bioengineering, 109(8), 1958–1964. https://doi.org/10.1002/bit.24477
Menegazzo, M. L., Nascimento, V. M., Hestekin, C. N., Hestekin, J. A., & Fonseca, G. G. (2020). Evaluation of Chlorella sorokiniana cultivated in outdoor photobioreactors for biodiesel production. Biofuels, 13(4), 483–488. https://doi.org/10.1080/17597269.2020.1763094
Darki, B. Z., Seyfabadi, J., & Fayazi, S. (2017). Effect of nutrients on total lipid content and fatty acids profile of Scenedesmus obliquus. Brazilian Archives of Biology and Technology. https://doi.org/10.1590/1678-4324-2017160304
Ma, C., Zhang, Y.-B., Ho, S.-H., Xing, D.-F., Ren, N.-Q., & Liu, B.-F. (2017). Cell growth and lipid accumulation of a microalgal mutant Scenedesmus sp. Z-4 by combining light/dark cycle with temperature variation. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-017-0948-0
Shao, Y., Fang, H., Zhou, H., Wang, Q., Zhu, Y., & He, Y. (2017). Detection and imaging of lipids of Scenedesmus obliquus based on confocal Raman Microspectroscopy. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-017-0977-8
Ren, H.-Y., Liu, B.-F., Ma, C., Zhao, L., & Ren, N.-Q. (2013). A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile Red staining: Effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnology for Biofuels, 6(1), 143. https://doi.org/10.1186/1754-6834-6-143
Bagchi, S. K., & Mallick, N. (2016). Carbon dioxide biofixation and lipid accumulation potential of an indigenous microalga Scenedesmus obliquus (turpin) Kützing GA 45 for biodiesel production. RSC Advances, 6(36), 29889–29898. https://doi.org/10.1039/c6ra02811j
Karpagam, R., Rani, K., Ashokkumar, B., Moorthy, I. G., Dhakshinamoorthy, A., & Varalakshmi, P. (2020). Green energy from Coelastrella sp. M-60: Bio-nanoparticles mediated whole biomass transesterification for biodiesel production. Fuel, 279, 118490. https://doi.org/10.1016/j.fuel.2020.118490
Angelaalincy, M., Nishtha, P., Ajithkumar, V., Ashokkumar, B., Moorthy, I. M. G., Brindhadevi, K., Chi, N. T. L., Pugazhendhi, A., & Varalakshmi, P. (2023). Phycoremediation of arsenic and biodiesel production using green microalgae Coelastrella sp. M60—an integrated approach. Fuel, 333, 126427. https://doi.org/10.1016/j.fuel.2022.126427
Nayana, K., Sudhakar, M. P., & Arunkumar, K. (2022). Biorefinery potential of Coelastrella biomass for fuel and bioproducts—a review. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-02519-9
Mansur, D., Fitriady, M. A., Susilaningsih, D., & Simanungkalit, S. P. (2017). Production of biodiesel from Coelastrella sp. microalgae. AIP Conference Proceedings, 10(1063/1), 5011925.
Minyuk, G., Chelebieva, E., Chubchikova, I., Dantsyuk, N., Drobetskaya, I., Sakhon, E., Chekanov, K., & Solovchenko, A. (2017). Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae, 32(3), 245–259. https://doi.org/10.4490/algae.2017.32.8.6
Abe, K., Hattori, H., & Hirano, M. (2007). Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chemistry, 100(2), 656–661. https://doi.org/10.1016/j.foodchem.2005.10.026
Rodríguez-Palacio, M. C., Cabrera-Cruz, R. B. E., Rolón-Aguilar, J. C., Tobías-Jaramillo, R., Martínez-Hernández, M., & Lozano-Ramírez, C. (2022). The cultivation of five microalgae species and their potential for biodiesel production. Energy, Sustainability and Society. https://doi.org/10.1186/s13705-022-00337-5
Ogbonna, I. O., Okpozu, O. O., Ikwebe, J., & Ogbonna, J. C. (2018). Utilisation of Desmodesmus subspicatus LC172266 for simultaneous remediation of cassava wastewater and accumulation of lipids for biodiesel production. Biofuels, 10(5), 657–664. https://doi.org/10.1080/17597269.2018.1426164
Abinandan, S., Subashchandrabose, S. R., Venkateswarlu, K., & Megharaj, M. (2020). Sustainable iron recovery and biodiesel yield by acid-adapted microalgae, Desmodesmus sp. MAS1 and Heterochlorella sp. MAS3, grown in synthetic acid mine drainage. ACS Omega, 5(12), 6888–6894. https://doi.org/10.1021/acsomega.0c00255
Vimali, E., Kumar, A. S., Vignesh, N. S., Ashokkumar, B., Dhakshinamoorthy, A., Udayan, A., Arumugam, M., Pugazhendhi, A., & Varalakshmi, P. (2022). Enhancement of lipid accumulation in microalga Desmodesmus sp. VV2: Response surface methodology and artificial neural network modeling for biodiesel production. Chemosphere, 293, 133477. https://doi.org/10.1016/j.chemosphere.2021.133477
Do, J.-M., Yeo, H.-T., Suh, H. S., & Yoon, H.-S. (2023). Effect of salt stress on the biomass productivity and potential bioenergy feedstock of Graesiella emersonii KNUA204 isolated from Ulleungdo Island. Frontiers in Energy Research. https://doi.org/10.3389/fenrg.2023.1056835
Kumar, V. S., Sarkar, S. D., Das, B. K., Sarkar, D. J., Gogoi, P., Maurye, P., Mitra, T., Talukder, A. K., Ganguly, S., Nag, S. K., Munilkumar, S., & Samanta, S. (2022). Sustainable biodiesel production from microalgae Graesiella emersonii through valorization of garden wastes-based vermicompost. Science of The Total Environment, 807, 150995. https://doi.org/10.1016/j.scitotenv.2021.150995
Wen, X., Du, K., Wang, Z., Peng, X., Luo, L., Tao, H., Xu, Y., Zhang, D., Geng, Y., & Li, Y. (2016). Effective cultivation of microalgae for biofuel production: A pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-016-0541-y
Xia, L., Yang, H., He, Q., & Hu, C. (2014). Physiological responses of freshwater oleaginous microalgae Desmodesmus sp. NMX-451 under nitrogen deficiency and alkaline pH-induced lipid accumulation. Journal of Applied Phycology, 27(2), 649–659. https://doi.org/10.1007/s10811-014-0371-x
Chaudhary, R., Khattar, J., & Singh, D. (2017). Growth and lipid production by Desmodesmus subspicatus and potential of lipids for biodiesel production. Journal of Energy and Environmental Sustainability, 4, 58–63.
Joe, M. H., Kim, D. H., Choi, D. S., & Bai, S. (2018). Optimization of phototrophic growth and lipid production of a newly isolated microalga, Desmodesmus sp. KAERI-NJ5. Microbiology and Biotechnology Letters, 46(4), 377–389. https://doi.org/10.4014/mbl.1808.08003
Xia, L., Ge, H., Zhou, X., Zhang, D., & Hu, C. (2013). Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus xj-15. Bioresource Technology, 144, 261–267. https://doi.org/10.1016/j.biortech.2013.06.112
Abdulsamad, J. K., Varghese, S. A., & Thajudeen, J. (2019). Cost effective cultivation and biomass production of green microalga Desmodesmus subspicatus MB 23 in NPK fertilizer medium. Journal of Microbiology, Biotechnology and Food Sciences, 9(3), 599–604.https://doi.org/10.15414/jmbfs.2019/20.9.3.599-604
Ho, S.-H., Chang, J.-S., Lai, Y.-Y., & Chen, C.-N.N. (2014). Achieving high lipid productivity of a thermotolerant microalga Desmodesmus sp. F2 by optimizing environmental factors and nutrient conditions. Bioresource Technology, 156, 108–116. https://doi.org/10.1016/j.biortech.2014.01.017
Arguelles, E., Laurena, A. C., Monsalud, R. G., & Martinez-Goss, M. R. (2019). High-lipid and protein-producing epilithic microalga, Desmodesmus sp. (U-AU2): A promising alternative feedstock for biodiesel and animal feed production. Philippine Journal of Crop Science, 44(2), 13–23.
Mandal, M. K., & Chaurasia, N. (2021). De novo transcriptomic analysis of Graesiella emersonii NC-M1 reveals differential genes expression in cell proliferation and lipid production under glucose and salt supplemented condition. Renewable Energy, 179, 2004–2015. https://doi.org/10.1016/j.renene.2021.07.141
Gara-Ali, M., Zili, F., Hosni, K., Ouada, H. B., & Ben-Mahrez, K. (2021). Lipophilic extracts of the thermophilic cyanobacterium Leptolyngbya sp. and chlorophyte Graesiella sp. and their potential use as food and anticancer agents. Algal Research, 60, 102511. https://doi.org/10.1016/j.algal.2021.102511
Cheng, W.-L., Shao, X.-M., Song, C.-F., Shi, F.-F., Ji, C.-L., & Li, R.-Z. (2017). Effects of nitrogen stress on growth and oil accumulation of Chlorella emersionii. Biotechnology Bulletin, 33(11), 160.
Mandal, M. K., Saikia, P., Chanu, N. K., & Chaurasia, N. (2019). Modulation of lipid content and lipid profile by supplementation of iron, zinc, and molybdenum in indigenous microalgae. Environmental Science and Pollution Research, 26(20), 20815–20828. https://doi.org/10.1007/s11356-019-05065-6
Jo, S.-W., Kang, N. S., Lee, J. A., Kim, E. S., Kim, K. M., Yoon, M., Hong, J. W., & Yoon, H.-S. (2020). Characterization of MABIK microalgae with biotechnological potentials. Journal of Marine Bioscience and Biotechnology, 12(1), 40–49.
Zili, F., Bouzidi, N., Ammar, J., Zakhama, W., Ghoul, M., Sayadi, S., & Ouada, H. B. (2016). Mixotrophic cultivation promotes growth, lipid productivity, and PUFA production of a thermophilic Chlorophyta strain related to the genus Graesiella. Journal of Applied Phycology, 29(1), 35–43. https://doi.org/10.1007/s10811-016-0941-1
Andrew, A. R., Yong, W. T. L., Misson, M., Anton, A., & Chin, G. J. W. L. (2022). Selection of tropical microalgae species for mass production based on lipid and fatty acid profiles. Frontiers in Energy Research. https://doi.org/10.3389/fenrg.2022.912904
Najeeb, M. I., Ahmad, M.-D., Anjum, A. A., Maqbool, A., Ali, M. A., Nawaz, M., Ali, T., & Manzoor, R. (2024). Distribution, screening and biochemical characterization of indigenous microalgae for bio-mass and bio-energy production potential from three districts of Pakistan. Brazilian Journal of Biology. https://doi.org/10.1590/1519-6984.261698
Ogbonna, I. O., Ikwebe, J., Okpozu, O. O., Eze, C. N., & Ogbonna, J. C. (2021). Potential of Dictyosphaerium sp. LC172264 concomitant remediation of cassava wastewater and accumulation of lipids for biodiesel production. Advances in Bioscience and Biotechnology, 12(08), 257–274. https://doi.org/10.4236/abb.2021.128016
Nayana, B., Venkatesh, H. N., Sudharshana, T. N., Manjunath, K., & Mohana, D. C. (2018). Isolation and utilization of Dictyosphaerium ehrenbergianum nageli for biodiesel production: Lipid extraction and biodiesel property analysis. Biofuels, 11(8), 885–892. https://doi.org/10.1080/17597269.2018.1432270
Jiang, Y., Pu, X., Zheng, D., Zhu, T., Wang, S., Deng, L., & Wang, W. (2018). Cultivation of lipid-producing microalgae in struvite-precipitated liquid digestate for biodiesel production. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-018-1102-3
Asoiro, F. U., Okonkwo, W. I., & Nweze, N. O. (2019). Studies on the growth rate, oil yield and properties of some indigenous freshwater microalgae species. Journal of Environmental Science and Technology, 12(4), 164–176. https://doi.org/10.3923/jest.2019.164.176
Cho, D.-H., Choi, J.-W., Kang, Z., Kim, B.-H., Oh, H.-M., Kim, H.-S., & Ramanan, R. (2017). Microalgal diversity fosters stable biomass productivity in open ponds treating wastewater. Scientific Reports. https://doi.org/10.1038/s41598-017-02139-8
Selvarajan, R., Felföldi, T., Tauber, T., Sanniyasi, E., Sibanda, T., & Tekere, M. (2015). Screening and evaluation of some green algal strains (Chlorophyceae) isolated from freshwater and soda lakes for biofuel production. Energies, 8(7), 7502–7521. https://doi.org/10.3390/en8077502
Huang, S. T., Goh, J. L., Ahmadzadeh, H., & Murry, M. A. (2019). A rapid sampling technique for isolating highly productive lipid-rich algae strains from environmental samples. Biofuel Research Journal, 6(1), 920–926. https://doi.org/10.18331/brj2019.6.1.3
Severes, A., Hegde, S., D’Souza, L., & Hegde, S. (2017). Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae based biofuels. Journal of Photochemistry and Photobiology B: Biology, 170, 235–240. https://doi.org/10.1016/j.jphotobiol.2017.04.023
Nishchal, G. S., & Goud, V. V. (2017). Salinity induced lipid production in microalgae and cluster analysis (ICCB 16-BR_047). Bioresource Technology, 242, 244–252. https://doi.org/10.1016/j.biortech.2017.03.175
Přibyl, P., Cepák, V., & Zachleder, V. (2012). Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Applied Microbiology and Biotechnology, 94(2), 549–561. https://doi.org/10.1007/s00253-012-3915-5
Mujtaba, G., Choi, W., Lee, C.-G., & Lee, K. (2012). Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresource Technology, 123, 279–283. https://doi.org/10.1016/j.biortech.2012.07.057
He, P. J., Mao, B., Shen, C. M., Shao, L. M., Lee, D. J., & Chang, J. S. (2013). Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresource Technology, 129, 177–181. https://doi.org/10.1016/j.biortech.2012.10.162
Kumar, A., Ergas, S., Yuan, X., Sahu, A., Zhang, Q., Dewulf, J., Malcata, F. X., & van Langenhove, H. (2010). Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends in Biotechnology, 28(7), 371–380. https://doi.org/10.1016/j.tibtech.2010.04.004
An, M., Gao, L., Zhao, W., Chen, W., & Li, M. (2020). Effects of nitrogen forms and supply mode on lipid production of microalga Scenedesmus obliquus. Energies, 13(3), 697. https://doi.org/10.3390/en13030697
Difusa, A., Talukdar, J., Kalita, M. C., Mohanty, K., & Goud, V. V. (2015). Effect of light intensity and pH condition on the growth, biomass and lipid content of microalgae Scenedesmus species. Biofuels, 6(1–2), 37–44. https://doi.org/10.1080/17597269.2015.1045274
El-Sheekh, M., Abomohra, A.E.-F., Abd El-Azim, M., & Abou-Shanab, R. (2017). Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga Scenedesmus acutus. BASE, 21(4), 233–239.
Pancha, I., Chokshi, K., George, B., Ghosh, T., Paliwal, C., Maurya, R., & Mishra, S. (2014). Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresource Technology, 156, 146–154. https://doi.org/10.1016/j.biortech.2014.01.025
Ogbonna, I. O., Ikwebe, J., Ogbonna, J. C., Eze, C. N., & Ndrimbula, J. B. (2022). Effects of light intensity and photoperiod on growth, lipid accumulation and fatty acid composition of Desmodesmus subspicatus LC172266 under photoautotrophic cultivation. Nigerian Journal of Biotechnology, 38(2), 1–13. https://doi.org/10.4314/njb.v38i2.1
Nzayisenga, J. C., Farge, X., Groll, S. L., & Sellstedt, A. (2020). Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-019-1646-x
Maltsev, Y., Krivova, Z., Maltseva, S., Maltseva, K., Gorshkova, E., & Kulikovskiy, M. (2021). Lipid accumulation by Coelastrella multistriata (Scenedesmaceae, Sphaeropleales) during nitrogen and phosphorus starvation. Scientific Reports. https://doi.org/10.1038/s41598-021-99376-9
Yao, L., Gerde, J. A., Lee, S.-L., Wang, T., & Harrata, K. A. (2015). Microalgae lipid characterization. Journal of Agricultural and Food Chemistry, 63(6), 1773–1787. https://doi.org/10.1021/jf5050603
Krzemińska, I., Nosalewicz, A., Reszczyńska, E., & Pawlik-Skowrońska, B. (2020). Enhanced light-induced biosynthesis of fatty acids suitable for biodiesel production by the yellow-green alga Eustigmatos magnus. Energies, 13(22), 6098. https://doi.org/10.3390/en13226098
Kostik, V., Memeti, S., & Bauer, B. (2013). Fatty acid composition of edible oils and fats. Journal of Hygienic Engineering and Design, 4, 112–116.
Aslam, A., Thomas-Hall, S. R., Manzoor, M., Jabeen, F., Iqbal, M., uz Zaman, Q., Schenk, P. M., & Tahir, M. A. (2018). Mixed microalgae consortia growth under higher concentration of CO2 from unfiltered coal-fired flue gas: Fatty acid profiling and biodiesel production. Journal of Photochemistry and Photobiology B: Biology, 179, 126–133. https://doi.org/10.1016/j.jphotobiol.2018.01.003
Patel, A., Arora, N., Mehtani, J., Pruthi, V., & Pruthi, P. A. (2017). Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renewable and Sustainable Energy Reviews, 77, 604–616. https://doi.org/10.1016/j.rser.2017.04.016
Sharma, V., Hossain, A., & Duraisamy, G. (2021). Experimental investigation of neat biodiesels’ saturation level on combustion and emission characteristics in a CI engine. Energies, 14(16), 5203. https://doi.org/10.3390/en14165203
Helwani, Z., Zahrina, I., Yelmida, Neonufa, G., Syamsuddin, Y., Rahmasari, A., Othman, M. R., & Idroes, R. (2023). Production of high-performance biodiesel with a high oxidation stability through a fractionation method using urea. South African Journal of Chemical Engineering, 45, 162–171. https://doi.org/10.1016/j.sajce.2023.05.009
Lucakova, S., Branyikova, I., & Hayes, M. (2022). Microalgal proteins and bioactives for food, feed, and other applications. Applied Sciences, 12(9), 4402. https://doi.org/10.3390/app12094402
Amorim, M. L., Soares, J., dos Reis Coimbra, J. S., de Oliveira Leite, M., Albino, L. F. T., & Martins, M. A. (2020). Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Critical Reviews in Food Science and Nutrition, 61(12), 1976–2002. https://doi.org/10.1080/10408398.2020.1768046
Barka, A., & Blecker, C. (2016). Microalgae as a potential source of single-cell proteins. A review. BASE. https://doi.org/10.25518/1780-4507.13132
Apandi, N. M., Mohamed, R. M. S. R., Latiffi, N. A. A., Rozlan, N. F. M., & Al-Gheethi, A. A. S. (2017). Protein and lipid content of microalgae Scenedesmus sp. biomass grown in wet market wastewater. MATEC Web of Conferences, 103, 06011. https://doi.org/10.1051/matecconf/201710306011
Duong, V. T., Ahmed, F., Thomas-Hall, S. R., Quigley, S., Nowak, E., & Schenk, P. M. (2015). High protein and high lipid-producing microalgae from Northern Australia as potential feedstock for animal feed and biodiesel. Frontiers in Bioengineering and Biotechnology. https://doi.org/10.3389/fbioe.2015.00053
Leyla, U., Oya, I., Bariş, Y., & Sayin, S. (2022). Effects of nitrogen and phosphorus concentrations on the growth and lipid accumulation of microalgae Scenedesmus obliquus. Marine Science and Technology Bulletin, 11(2), 194–201.
Adenan, N. S., Yusoff, F. M., Medipally, S. R., & Shariff, M. (2016). Enhancement of lipid production in two marine microalgae under different levels of nitrogen and phosphorus deficiency. Journal of Environmental Biology, 37(4), 669.
Eze, C. N., Ogbonna, I. O., & Ogbonna, J. C. (2022). Growth, lipids, proteins, and carotenoid contents of some freshwater green microalgae under simulated day/night temperature fluctuation. Nigerian Journal of Biotechnology, 39(1), 9–19. https://doi.org/10.4314/njb.v39i1.2
Asfouri, N. Y., Hamed, M., Abi-Ayad, S., & Lamara, S. (2019). The influence of light intensity and photoperiod on the growth of two fresh water green algae Tetranephris brasiliensis and Scenedesmus sp. isolated from estuary. Indian Journal of Research, 15(3), 192.
Seyfabadi, J., Ramezanpour, Z., & Khoeyi, Z. A. (2010). Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. Journal of Applied Phycology, 23(4), 721–726. https://doi.org/10.1007/s10811-010-9569-8
Qiu, R., Gao, S., Lopez, P. A., & Ogden, K. L. (2017). Effects of pH on cell growth, lipid production and CO\(_2\) addition of microalgae Chlorella sorokiniana. Algal Research, 28, 192–199. https://doi.org/10.1016/j.algal.2017.11.004
Liang, K., Zhang, Q., Gu, M., & Cong, W. (2012). Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. Journal of Applied Phycology, 25(1), 311–318. https://doi.org/10.1007/s10811-012-9865-6
Chandra, N., Shukla, P., & Mallick, N. (2020). Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatalysis and Agricultural Biotechnology, 26, 101632. https://doi.org/10.1016/j.bcab.2020.101632
Wang, Y., Xu, H., Yang, J., Zhou, Y., Wang, X., Dou, S., Li, L., Liu, G., & Yang, M. (2022). Effect of sulfur limitation strategies on glucose-based carbohydrate production from Chlorella sorokiniana. Renewable Energy, 200, 449–456. https://doi.org/10.1016/j.renene.2022.09.106
Bremauntz, P., Fernández-Linares, L. C., & Cañizares-Villanueva, R. O. (2014). Osmotic stress effect over carbohydrate production in a native strain of Scenedesmus sp. Natural Resources, 05(01), 5–9. https://doi.org/10.4236/nr.2014.51002
Ho, S.-H., Chen, C.-Y., & Chang, J.-S. (2012). Effect of light intensity and nitrogen starvation on CO\(_2\) fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresource Technology, 113, 244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Pancha, I., Chokshi, K., Maurya, R., Trivedi, K., Patidar, S. K., Ghosh, A., & Mishra, S. (2015). Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresource Technology, 189, 341–348. https://doi.org/10.1016/j.biortech.2015.04.017
Militão, F. P., de Oliveira Fernandes, V., Bastos, K. V., Martins, A. P., Colepicolo, P., & Machado, L. P. (2019). Nutritional value changes in response to temperature, microalgae mono and mixed cultures. Acta Limnologica Brasiliensia. https://doi.org/10.1590/s2179-975x7118
Reungsang, A., & Plangklang, P. (2023). Valorization of cassava ethanol waste as carbon and nutrient sources for microalgae cultivation. Asia-Pacific Journal of Science and Technology, 100, 100. https://doi.org/10.14456/apst.2023.54
Huo, S., Wang, Z., Cui, F., Zou, B., Zhao, P., & Yuan, Z. (2015). Enzyme-assisted extraction of oil from wet microalgae Scenedesmus sp. g4. Energies, 8(8), 8165–8174. https://doi.org/10.3390/en8088165
Zhang, Y., Ding, Z., Hossain, M. S., Maurya, R., Yang, Y., Singh, V., Kumar, D., Salama, E.-S., Sun, X., Sindhu, R., Binod, P., Zhang, Z., & Awasthi, M. K. (2023). Recent advances in lignocellulosic and algal biomass pretreatment and its biorefinery approaches for biochemicals and bioenergy conversion. Bioresource Technology, 367, 128281. https://doi.org/10.1016/j.biortech.2022.128281
Sarwer, A., Hamed, S. M., Osman, A. I., Jamil, F., Al-Muhtaseb, A. H., Alhajeri, N. S., & Rooney, D. W. (2022). Algal biomass valorization for biofuel production and carbon sequestration: A review. Environmental Chemistry Letters, 20(5), 2797–2851. https://doi.org/10.1007/s10311-022-01458-1
Zhang, A., Fang, S., Xi, H., Huang, J., Li, Y., Ma, G., & Zhang, J. (2023). Highly efficient and selective removal of phosphate from wastewater of sea cucumber aquaculture for microalgae culture using a new adsorption-membrane separation-coordinated strategy. Frontiers of Environmental Science & Engineering. https://doi.org/10.1007/s11783-023-1720-2
Aketo, T., Waga, K., Yabu, Y., Maeda, Y., Yoshino, T., Hanada, A., Sano, K., Kamiya, T., Takano, H., & Tanaka, T. (2021). Algal biomass production by phosphorus recovery and recycling from wastewater using amorphous calcium silicate hydrates. Bioresource Technology, 340, 125678. https://doi.org/10.1016/j.biortech.2021.125678
Baykalözer, T., & Aydin Akbulut, A. U. U. (2012). Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (turpin) kützing 1833. African Journal of Biotechnology. https://doi.org/10.5897/ajb11.1863
Sukumaran, S. (2017). Protein secondary structure elucidation using FTIR spectroscopy. Thermo Fisher Scientific.
Shao, Y., Gu, W., ating Qiu, Y., Wang, S., Peng, Y., Zhu, Y., & Zhuang, S. (2020). Lipids monitoring in Scenedesmus obliquus based on terahertz technology. Biotechnology for Biofuels. https://doi.org/10.1186/s13068-020-01801-0
Acknowledgements
Authors of this work thanks CSIR, Govt. of India for financial support in the form of fellowship and STEP, TIET for laboratory support.
Author information
Authors and Affiliations
Contributions
NH, RKA, and DG conceived and designed the study. NH performed the data collection. NH and DG analyzed the data. All authors contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest or competing interests that could influence the research findings or bias the interpretation of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Halder, N., Goyal, D. & Aneja, R.K. Bioprospecting Microalgae from Sewage Water: Assessment of Biochemicals for Biomass Utilization. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00969-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00969-8