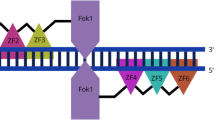
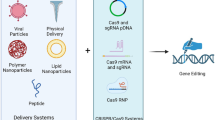
Abstract
Gene mutation correction was challenging until the discovery of clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein (Cas). CRISPR is a new era for genome modification, and this technology has bypassed the limitations of previous methods such as zinc-finger nuclease and transcription activator-like effector nuclease. Currently, this method is becoming the method of choice for gene-editing purposes, especially therapeutic gene editing in diseases such as cardiovascular, neurological, renal, genetic, optical, and stem cell, as well as blood disorders and muscular degeneration. However, finding the optimum delivery system capable of carrying this large complex persists as the main challenge of this technology. Therefore, it would be ideal if the delivery vehicle could direct the introduction of editing functions to specific cells in a multicellular organism. Exosomes are membrane-bound vesicles with high biocompatibility and low immunogenicity; they offer the best and most reliable way to fill the CRISPR/Cas9 system delivery gap. This review presents the current evidence on the molecular mechanisms and challenges of CRISPR/Cas9-mediated genome modification. Also, the role of CRISPR/Cas9 in the development of treatment and diagnosis of numerous disorders, from malignancies to viral infections, has been discussed. Lastly, the focus is on new advances in exosome-delivery technologies that may play a role in CRISPR/Cas9 delivery for future clinical settings.
Graphical Abstract




Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Kim, T. H., & Lee, S. W. (2022). Therapeutic application of genome editing technologies in viral diseases. International Journal of Molecular Sciences., 23(10), 5399.
Bhattacharjee, G., et al. (2020). CRISPR technology for genome editing. Precision medicine for investigators, practitioners and providers (pp. 59–69). Elsevier.
Li, H., et al. (2020). Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal transduction and targeted therapy, 5(1), 1.
Hsu, P. D., Lander, E. S., & Zhang, F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell, 157(6), 1262–1278.
Newsom, S., Parameshwaran, H. P., Martin, L., & Rajan, R. (2021). The CRISPR-Cas mechanism for adaptive immunity and alternate bacterial functions fuels diverse biotechnologies. Frontiers in Cellular and Infection Microbiology, 10, 619763.
Redman, M., et al. (2016). What is CRISPR/Cas9? Archives of Disease in Childhood-Education and Practice, 101(4), 213–215.
Xu, Y., & Li, Z. (2020). CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Computational and Structural Biotechnology Journal., 18, 2401–15.
Huang, J., Zhou, Y., Li, J., Lu, A., & Liang, C. (2022). CRISPR/Cas systems: Delivery and application in gene therapy. Frontiers in Bioengineering and Biotechnology., 22(10), 942325.
Li, Y., Li, S., Wang, J., & Liu, G. (2019). CRISPR/Cas systems towards next-generation biosensing. Trends in Biotechnology, 37(7), 730–743.
He, Y., Yan, W., Long, L., Dong, L., Ma, Y., Li, C., Xie, Y., Liu, N., Xing, Z., Xia, W., & Li, F. (2023). The CRISPR/Cas system: A customizable toolbox for molecular detection. Genes, 14(4), 850.
Frangoul, H., Altshuler, D., Cappellini, M. D., Chen, Y. S., Domm, J., Eustace, B. K., Foell, J., de la Fuente, J., Grupp, S., Handgretinger, R., & Ho, T. W. (2021). CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. New England Journal of Medicine, 384(3), 252–60.
Liu, W., Li, L., Jiang, J., Wu, M., & Lin, P. (2021). Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precision Clinical Medicine., 4(3), 179–91.
Duan, L., et al. (2021). Exosomes as targeted delivery platform of CRISPR/Cas9 for therapeutic genome editing. ChemBioChem, 22(24), 3360–3368.
Johnsen, K. B., et al. (2014). A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1846(1), 75–87.
Trams, E. G., et al. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et Biophysica Acta (BBA)-Biomembranes, 645(1), 63–70.
Johnstone, R. M., et al. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). Journal of Biological Chemistry, 262(19), 9412–9420.
Shao, J., Zaro, J., & Shen, Y. (2020). Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. International Journal of Nanomedicine, 15, 9355–9371.
Ishino, Y., et al. (1987). Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. Journal of Bacteriology, 169(12), 5429–5433.
Mojica, F. J., et al. (2000). Biological significance of a family of regularly spaced repeats in the genomes of Archaea, bacteria and mitochondria. Molecular Microbiology, 36(1), 244–246.
Bolotin, A., et al. (2005). Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology, 151(8), 2551–2561.
Tang, H., Zhao, X., & Jiang, X. (2021). Synthetic multi-layer nanoparticles for CRISPR-Cas9 genome editing. Advanced Drug Delivery Reviews, 168, 55–78.
Barrangou, R., et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science, 315(5819), 1709–1712.
Brouns, S. J., et al. (2008). Small CRISPR RNAs guide antiviral defense in prokaryotes. Science, 321(5891), 960–964.
Marraffini, L. A., & Sontheimer, E. J. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science, 322(5909), 1843–1845.
Marraffini, L. A., & Sontheimer, E. J. (2010). CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nature Reviews Genetics, 11(3), 181–190.
Deltcheva, E., et al. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 471(7340), 602–607.
Haft, D. H., et al. (2005). A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Computational Biology, 1(6), e60.
Makarova, K. S., et al. (2011). Evolution and classification of the CRISPR–Cas systems. Nature Reviews Microbiology, 9(6), 467–477.
Zhang, F., Wen, Y., & Guo, X. (2014). CRISPR/Cas9 for genome editing: Progress, implications and challenges. Human Molecular Genetics, 23(R1), R40–R46.
Feng, Y., et al. (2021). Target binding and residence: A new determinant of DNA double-strand break repair pathway choice in CRISPR/Cas9 genome editing. Journal of Zhejiang University-Science B, 22(1), 73–86.
Jinek, M., et al. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816–821.
Mali, P., et al. (2013). RNA-guided human genome engineering via Cas9. Science, 339(6121), 823–826.
Cong, L., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science, 339(6121), 819–823.
Doudna, J. A., & Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science, 346(6213), 1258096.
Liu, C., et al. (2017). Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. Journal of Controlled release, 266, 17–26.
Jiang, F., & Doudna, J. A. (2017). CRISPR–Cas9 structures and mechanisms. Annual review of Biophysics, 46, 505–529.
Cox, D. B. T., Platt, R. J., & Zhang, F. (2015). Therapeutic genome editing: Prospects and challenges. Nature Medicine, 21(2), 121–131.
Sander, J. D., & Joung, J. K. (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology, 32(4), 347–355.
Abudayyeh, O. O., et al. (2017). RNA targeting with CRISPR–Cas13. Nature, 550(7675), 280–284.
Merkle, T., et al. (2019). Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nature Biotechnology, 37(2), 133–138.
Tucker, B. A., et al. (2011). Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE, 6(4), e18992.
Homma, K., et al. (2013). Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors. Stem Cells, 31(6), 1149–1159.
Liang, P., et al. (2015). CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & Cell, 6(5), 363–372.
Khan, S., et al. (2018). CRISPR/Cas9: The Jedi against the dark empire of diseases. Journal of Biomedical Science, 25(1), 1–18.
Cai, L., et al. (2016). CRISPR-mediated genome editing and human diseases. Genes & Diseases, 3(4), 244–251.
Kolli, N., et al. (2018). Application of the gene editing tool, CRISPR-Cas9, for treating neurodegenerative diseases. Neurochemistry International, 112, 187–196.
Zhang, H., et al. (2021). Interaction between Aβ and tau in the pathogenesis of Alzheimer’s disease. International Journal of Biological Sciences, 17(9), 2181.
Park, H., et al. (2019). In vivo neuronal gene editing via CRISPR–Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer’s disease. Nature Neuroscience, 22(4), 524–528.
He, G., et al. (2010). Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature, 467(7311), 95–98.
Wong, E., et al. (2019). GSAP modulates γ-secretase specificity by inducing conformational change in PS1. Proceedings of the National Academy of Sciences, 116(13), 6385–6390.
György, B., et al. (2018). CRISPR/Cas9 mediated disruption of the Swedish APP allele as a therapeutic approach for early-onset Alzheimer’s disease. Molecular Therapy-Nucleic Acids, 11, 429–440.
Nagata, K., et al. (2018). Generation of App knock-in mice reveals deletion mutations protective against Alzheimer’s disease-like pathology. Nature Communications, 9(1), 1–7.
Lee, J. K., & Kim, N.-J. (2017). Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules, 22(8), 1287.
Karimian, A., et al. (2020). CRISPR/Cas9 novel therapeutic road for the treatment of neurodegenerative diseases. Life Sciences, 259, 118165.
Arias-Fuenzalida, J., et al. (2017). FACS-assisted CRISPR-Cas9 genome editing facilitates Parkinson’s disease modeling. Stem Cell Reports, 9(5), 1423–1431.
Qing, X., et al. (2017). CRISPR/Cas9 and piggyBac-mediated footprint-free LRRK2-G2019S knock-in reveals neuronal complexity phenotypes and α-Synuclein modulation in dopaminergic neurons. Stem Cell Research, 24, 44–50.
Song, C., et al. (2019). Mechanistic interplay between autophagy and apoptotic signaling in endosulfan-induced dopaminergic neurotoxicity: Relevance to the adverse outcome pathway in pesticide neurotoxicity. Toxicological Sciences, 169(2), 333–352.
Gordon, R., et al. (2016). Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration. Nature Communications, 7(1), 1–18.
Kempuraj, D., et al. (2013). Glia maturation factor induces interleukin-33 release from astrocytes: Implications for neurodegenerative diseases. Journal of Neuroimmune Pharmacology, 8(3), 643–650.
Selvakumar, G. P., et al. (2019). CRISPR/Cas9 editing of glia maturation factor regulates mitochondrial dynamics by attenuation of the NRF2/HO-1 dependent ferritin activation in glial cells. Journal of Neuroimmune Pharmacology, 14, 537–550.
Wu, J., et al. (2018). Inhibition of TRPC1-dependent store-operated calcium entry improves synaptic stability and motor performance in a mouse model of Huntington’s disease. Journal of Huntington’s Disease, 7(1), 35–50.
Lee, J., et al. (2002). An upstream open reading frame impedes translation of the huntingtin gene. Nucleic Acids Research, 30(23), 5110–5119.
Kolli, N., et al. (2017). CRISPR-Cas9 mediated gene-silencing of the mutant huntingtin gene in an in vitro model of Huntington’s disease. International Journal of Molecular Sciences, 18(4), 754.
Chemello, F., Bassel-Duby, R., & Olson, E. N. (2020). Correction of muscular dystrophies by CRISPR gene editing. The Journal of Clinical Investigation, 130(6), 2766–2776.
Li, J., et al. (2020). CRISPR/Cas9-mediated miR-29b editing as a treatment of different types of muscle atrophy in mice. Molecular Therapy, 28(5), 1359–1372.
Long, C., et al. (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science, 351(6271), 400–403.
Nelson, C. E., et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science, 351(6271), 403–407.
Li, H. L., et al. (2015). Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports, 4(1), 143–154.
Men, K., et al. (2017). CRISPR/Cas9-mediated correction of human genetic disease. Science China Life Sciences, 60(5), 447–457.
Ousterout, D. G., et al. (2015). Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nature Communications, 6(1), 1–13.
Xu, L., et al. (2016). CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Molecular Therapy, 24(3), 564–569.
Xiong, X., et al. (2016). CRISPR/Cas9 for human genome engineering and disease research. Annual Review of Genomics and Human Genetics, 17, 131–154.
Abboud, S., et al. (2007). Proprotein convertase subtilisin/kexin type 9 (PCSK9) gene is a risk factor of large-vessel atherosclerosis stroke. PLoS ONE, 2(10), e1043.
Zhang, L., et al. (2019). Triple-targeting delivery of CRISPR/Cas9 To reduce the risk of cardiovascular diseases. Angewandte Chemie International Edition, 58(36), 12404–12408.
Motta, B.M., et al., The impact of CRISPR/Cas9 technology on cardiac research: from disease modelling to therapeutic approaches. Stem cells international, 2017. 2017.
Yamamoto, Y., et al. (2017). Allele-specific ablation rescues electrophysiological abnormalities in a human iPS cell model of long-QT syndrome with a CALM2 mutation. Human Molecular Genetics, 26(9), 1670–1677.
Rezaei, H., et al. (2020). Harnessing CRISPR/Cas9 technology in cardiovascular disease. Trends in Cardiovascular Medicine, 30(2), 93–101.
Limpitikul, W. B., et al. (2017). A precision medicine approach to the rescue of function on malignant calmodulinopathic long-QT syndrome. Circulation Research, 120(1), 39–48.
Zabaleta, N., et al. (2018). CRISPR/Cas9-mediated glycolate oxidase disruption is an efficacious and safe treatment for primary hyperoxaluria type I. Nature Communications, 9(1), 1–9.
Humbert, O., Samuelson, C., & Kiem, H. P. (2021). CRISPR/Cas9 for the treatment of haematological diseases: A journey from bacteria to the bedside. British Journal of Haematology, 192(1), 33–49.
Ye, L., et al. (2016). Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proceedings of the National Academy of Sciences, 113(38), 10661–10665.
Rodríguez-Rodríguez, D. R., et al. (2019). Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases. International Journal of Molecular Medicine, 43(4), 1559–1574.
Zhang, H., & McCarty, N. (2016). CRISPR-Cas9 technology and its application in haematological disorders. British Journal of Haematology, 175(2), 208–225.
Canver, M. C., et al. (2015). BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature, 527(7577), 192–197.
Xie, F., et al. (2014). Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Research, 24(9), 1526–1533.
Huang, X., et al. (2015). Production of gene-corrected adult beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells, 33(5), 1470–1479.
Renneville, A., et al. (2015). EHMT1 and EHMT2 inhibition induces fetal hemoglobin expression. Blood, The Journal of the American Society of Hematology, 126(16), 1930–1939.
Dever, D. P., et al. (2016). CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature, 539(7629), 384–389.
Traxler, E. A., et al. (2016). A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nature Medicine, 22(9), 987–990.
Osborn, M. J., et al. (2015). Fanconi anemia gene editing by the CRISPR/Cas9 system. Human Gene Therapy, 26(2), 114–126.
Ablain, J., et al. (2015). A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Developmental Cell, 32(6), 756–764.
Park, C.-Y., et al. (2015). Functional correction of large factor VIII gene chromosomal inversions in hemophilia A patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell, 17(2), 213–220.
Guan, Y., et al. (2016). CRISPR/Cas9-mediated somatic correction of a novel coagulator factor IX gene mutation ameliorates hemophilia in mouse. EMBO Molecular Medicine, 8(5), 477–488.
Chen, M., et al. (2019). CRISPR-Cas9 for cancer therapy: Opportunities and challenges. Cancer Letters, 447, 48–55.
Liu, B., Saber, A., & Haisma, H. J. (2019). CRISPR/Cas9: A powerful tool for identification of new targets for cancer treatment. Drug Discovery Today, 24(4), 955–970.
Cheung, A.H.-K., et al. (2018). Specific targeting of point mutations in EGFR L858R-positive lung cancer by CRISPR/Cas9. Laboratory Investigation, 98(7), 968–976.
Koo, T., et al. (2017). Selective disruption of an oncogenic mutant allele by CRISPR/Cas9 induces efficient tumor regression. Nucleic acids research, 45(13), 7897–7908.
Mou, H., et al. (2017). Genetic disruption of oncogenic Kras sensitizes lung cancer cells to Fas receptor-mediated apoptosis. Proceedings of the National Academy of Sciences, 114(14), 3648–3653.
Bu, X., et al. (2018). CD38 knockout suppresses tumorigenesis in mice and clonogenic growth of human lung cancer cells. Carcinogenesis, 39(2), 242–251.
Shen, F., et al. (2016). Novel small-molecule CX3CR1 antagonist impairs metastatic seeding and colonization of breast cancer cells. Molecular Cancer Research, 14(6), 518–527.
Jin, K., Pandey, N. B., & Popel, A. S. (2017). Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget, 8(36), 60210.
Álvarez-Fernández, M., et al. (2017). Therapeutic relevance of the PP2A-B55 inhibitory kinase MASTL/Greatwall in breast cancer. Cell Death & Differentiation. https://doi.org/10.1038/s41418-017-0024-0
Karn, V., et al. (2022). CRISPR/Cas9 system in breast cancer therapy: Advancement, limitations and future scope. Cancer Cell International, 22(1), 1–14.
Zhang, S., et al. (2017). Suppression of protein tyrosine phosphatase N23 predisposes to breast tumorigenesis via activation of FYN kinase. Genes & Development, 31(19), 1939–1957.
Zheng, Y.-Z., et al. (2018). PHF5A epigenetically inhibits apoptosis to promote breast cancer progression. Cancer Research, 78(12), 3190–3206.
Peng, H., et al. (2016). Over-expression of CHAF1A promotes cell proliferation and apoptosis resistance in glioblastoma cells via AKT/FOXO3a/Bim pathway. Biochemical and Biophysical Research Communications, 469(4), 1111–1116.
Leisegang, M. S., et al. (2017). Long noncoding RNA MANTIS facilitates endothelial angiogenic function. Circulation, 136(1), 65–79.
Wang, X., et al. (2017). CRISPR/Cas9-mediated genome engineering of CXCR4 decreases the malignancy of hepatocellular carcinoma cells in vitro and in vivo. Oncology Reports, 37(6), 3565–3571.
Zhu, B., et al. (2017). Knockout of the Nogo-B gene attenuates tumor growth and metastasis in hepatocellular carcinoma. Neoplasia, 19(7), 583–593.
Yau, E. H., et al. (2017). Genome-wide CRISPR screen for essential cell growth mediators in mutant KRAS colorectal cancers. Cancer Research, 77(22), 6330–6339.
Zhou, M., et al. (2018). Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. International Journal of Cancer, 143(4), 921–930.
Xia, D., et al. (2017). Knockout of MARCH2 inhibits the growth of HCT116 colon cancer cells by inducing endoplasmic reticulum stress. Cell Death & Disease, 8(7), e2957–e2957.
Wu, X.-Y., et al. (2017). Identification of RING-box 2 as a potential target for combating colorectal cancer growth and metastasis. American Journal of Cancer Research, 7(6), 1238.
Yoshida, K., et al. (2017). SNORA21–an oncogenic small nucleolar RNA, with a prognostic biomarker potential in human colorectal cancer. eBioMedicine, 22, 68–77.
Norouzi-Barough, L., et al. (2018). CRISPR/Cas9, a new approach to successful knockdown of ABCB1/P-glycoprotein and reversal of chemosensitivity in human epithelial ovarian cancer cell line. Iranian Journal of Basic Medical Sciences, 21(2), 181.
Kim, M. Y., et al. (2016). Engineering resistance to antigen-specific immunotherapy in normal hematopoietic stem cells by gene editing to enable targeting of acute myeloid leukemia. Blood, 128(22), 1000.
Chen, C., et al. (2014). MLL3 is a haploinsufficient 7q tumor suppressor in acute myeloid leukemia. Cancer Cell, 25(5), 652–665.
Kawamura, N., et al. (2015). CRISPR/Cas9-mediated gene knockout of NANOG and NANOGP8 decreases the malignant potential of prostate cancer cells. Oncotarget, 6(26), 22361.
Zhu, D., et al. (2018). Nanoparticles based on poly (β-Amino Ester) and HPV16-Targeting CRISPR/shRNA as potential drugs for HPV16-related cervical malignancy. Molecular Therapy, 26(10), 2443–2455.
Kennedy, E. M., et al. (2015). Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology, 476, 196–205.
Lin, S.-R., et al. (2014). The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Molecular Therapy-Nucleic Acids, 3, e186.
Li, H., et al. (2017). Removal of integrated hepatitis B virus DNA using CRISPR-Cas9. Frontiers in Cellular and Infection Microbiology, 7, 91.
Kitamura, K., et al. (2018). Flap endonuclease 1 is involved in cccDNA formation in the hepatitis B virus. PLoS Pathogens, 14(6), e1007124.
Kaminski, R., et al. (2016). Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Scientific reports, 6(1), 1–15.
Wang, Z., et al. (2016). CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Reports, 15(3), 481–489.
Zhang, Y., et al. (2015). CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Scientific Reports, 5(1), 1–14.
Saayman, S. M., et al. (2016). Potent and targeted activation of latent HIV-1 using the CRISPR/dCas9 activator complex. Molecular Therapy, 24(3), 488–498.
Wang, J., & Quake, S. R. (2014). RNA-guided endonuclease provides a therapeutic strategy to cure latent herpesviridae infection. Proceedings of the National Academy of Sciences, 111(36), 13157–13162.
Kennedy, E. M., et al. (2014). Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. Journal of Virology, 88(20), 11965–11972.
Zhen, S., et al. (2016). In vitro and in vivo synergistic therapeutic effect of cisplatin with human papillomavirus16 E6/E7 CRISPR/Cas9 on cervical cancer cell line. Translational Oncology, 9(6), 498–504.
Hammond, A., et al. (2016). A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nature Biotechnology, 34(1), 78–83.
Gantz, V. M., et al. (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proceedings of the National Academy of Sciences, 112(49), E6736–E6743.
Zhang, W.-W., & Matlashewski, G. (2015). CRISPR-Cas9-mediated genome editing in Leishmania donovani. MBio, 6(4), e00861-e915.
Latella, M. C., et al. (2016). In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina. Molecular Therapy-Nucleic Acids, 5, e389.
Cai, B., et al. (2018). Application of CRISPR/Cas9 technologies combined with iPSCs in the study and treatment of retinal degenerative diseases. Human Genetics, 137(9), 679–688.
Bakondi, B., et al. (2016). In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Molecular Therapy, 24(3), 556–563.
Kim, K., et al. (2017). Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Research, 27(3), 419–426.
Wu, Y., et al. (2013). Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell, 13(6), 659–662.
Wu, Y., et al. (2015). Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Research, 25(1), 67–79.
Gibson, G. J., & Yang, M. (2017). What rheumatologists need to know about CRISPR/Cas9. Nature Reviews Rheumatology, 13(4), 205–216.
Tang, S., et al. (2014). RasGRP3 limits Toll-like receptor-triggered inflammatory response in macrophages by activating Rap1 small GTPase. Nature Communications, 5(1), 1–14.
Jing, W., et al. (2015). CRISPR/CAS9-mediated genome editing of miRNA-155 inhibits proinflammatory cytokine production by RAW264. 7 cells. BioMed Research International. https://doi.org/10.1155/2015/326042
Chang, C.-W., et al. (2015). Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting. Cell reports, 12(10), 1668–1677.
Flynn, R., et al. (2015). CRISPR-mediated genotypic and phenotypic correction of a chronic granulomatous disease mutation in human iPS cells. Experimental Hematology, 43(10), 838–848.
Chu, H. W., et al. (2015). CRISPR–Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Therapy, 22(10), 822–829.
Kuo, C. Y., et al. (2015). Site specific gene correction of defects in CD40 ligand using the Crispr/Cas9 genome editing platform. Journal of Allergy and Clinical Immunology, 135(2), 17.
Zuccaro, M. V., et al. (2020). Allele-specific chromosome removal after Cas9 cleavage in human embryos. Cell, 183(6), 1650–1664.
Kang, X., et al. (2016). Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. Journal of Assisted Reproduction and Genetics, 33, 581–588.
Tang, L., et al. (2017). CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Molecular Genetics and Genomics, 292(3), 525–533.
Ma, H., et al. (2017). Correction of a pathogenic gene mutation in human embryos. Nature, 548(7668), 413–419.
Schenkwein, D., & Ylä-Herttuala, S. (2018). Gene editing of human embryos with CRISPR/Cas9: Great promise coupled with important caveats. Molecular Therapy, 26(3), 659–660.
Cyranoski, D., & Ledford, H. (2018). Genome-edited baby claim provokes international outcry. Nature, 563(7731), 607–609.
Hoffmann, E. R., & Roig, I. (2020). Cas9 in human embryos: On target but no repair. Cell, 183(6), 1464–1466.
Di, C., et al. (2018). Exosomes as drug carriers for clinical application. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup3), S564–S570.
Luan, X., et al. (2017). Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica, 38(6), 754–763.
Aslan, C., et al. (2019). Tumor-derived exosomes: Implication in angiogenesis and antiangiogenesis cancer therapy. Journal of Cellular Physiology, 234(10), 16885–16903.
Aslan, C., et al. (2021). Exosomes for mRNA delivery: A novel biotherapeutic strategy with hurdles and hope. BMC Biotechnology, 21, 1–12.
Jiang, X.-C., & Gao, J.-Q. (2017). Exosomes as novel bio-carriers for gene and drug delivery. International Journal of Pharmaceutics, 521(1–2), 167–175.
Alvarez-Erviti, L., et al. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29(4), 341–345.
Taylor, D. D., & Gercel-Taylor, C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncology, 110(1), 13–21.
Rezaie, J., et al. (2018). Exosomes and their application in biomedical field: Difficulties and advantages. Molecular Neurobiology, 55(4), 3372–3393.
Andaloussi, S. E., et al. (2013). Exosomes for targeted siRNA delivery across biological barriers. Advanced Drug Delivery Reviews, 65(3), 391–397.
McAndrews, K. M., et al. (2021). Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Science Alliance. https://doi.org/10.26508/lsa.202000875
Ye, Y., et al. (2020). An engineered exosome for delivering sgRNA: Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomaterials science, 8(10), 2966–2976.
Wilbie, D., Walther, J., & Mastrobattista, E. (2019). Delivery aspects of CRISPR/Cas for in vivo genome editing. Accounts of Chemical Research, 52(6), 1555–1564.
Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science, 367(6478), 6977.
Chen, R., et al. (2019). Friend or foe? Evidence indicates endogenous exosomes can deliver functional gRNA and Cas9 protein. Small (Weinheim an der Bergstrasse, Germany), 15(38), 1902686.
Duan, L., et al. (2021). Nanoparticle delivery of CRISPR/Cas9 for genome editing. Frontiers in Genetics, 12, 673286.
Kim, S. M., et al. (2017). Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. Journal of Controlled Release, 266, 8–16.
Luo, N., et al. (2021). Hepatic stellate cell reprogramming via exosome-mediated CRISPR/dCas9-VP64 delivery. Drug Delivery, 28(1), 10–18.
Zhuang, J., et al. (2020). Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic acids research, 48(16), 8870–8882.
Zhang, X., et al. (2020). Programmable extracellular vesicles for macromolecule delivery and genome modifications. Developmental Cell, 55(6), 784–801.
Yao, X., et al. (2021). Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. Journal of Extracellular Vesicles, 10(5), e12076.
Li, Z., et al. (2018). In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Letters, 19(1), 19–28.
Usman, W. M., et al. (2018). Efficient RNA drug delivery using red blood cell extracellular vesicles. Nature Communications, 9(1), 2359.
Yang, Z., et al. (2020). Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nature Biomedical Engineering, 4(1), 69–83.
Lin, Y., et al. (2018). Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Advanced Science, 5(4), 1700611.
Horodecka, K., & Düchler, M. (2021). CRISPR/Cas9: Principle, applications, and delivery through extracellular vesicles. International Journal of Molecular Sciences, 22(11), 6072.
Zhu, X., et al. (2023). The CRISPR/Cas9 system delivered by extracellular vesicles. Pharmaceutics, 15(3), 984.
Khalyfa, A., & Sanz-Rubio, D. (2021). The mystery of red blood cells extracellular vesicles in sleep apnea with metabolic dysfunction. International Journal of Molecular Sciences, 22(9), 4301.
Emam, S. E., et al. (2019). Cancer cell-type tropism is one of crucial determinants for the efficient systemic delivery of cancer cell-derived exosomes to tumor tissues. European Journal of Pharmaceutics and Biopharmaceutics, 145, 27–34.
Liang, Y., et al. (2021). Engineering exosomes for targeted drug delivery. Theranostics, 11(7), 3183.
Rajput, A., et al. (2022). Exosomes as new generation vehicles for drug delivery: Biomedical applications and future perspectives. Molecules, 27(21), 7289.
Funding
This study was not financially supported by any organization in the private or governmental section.
Author information
Authors and Affiliations
Contributions
Conception and manuscript design: RJ collection of data: CA, NMZ, FF, and RJ. Manuscript writing: CA, NMZ, FF, and RJ. Made important revisions and confirmed final revision: FF and RJ. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslan, C., Zolbanin, N.M., Faraji, F. et al. Exosomes for CRISPR-Cas9 Delivery: The Cutting Edge in Genome Editing. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00932-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00932-7