Abstract
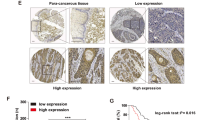
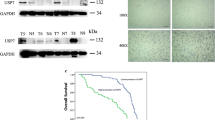
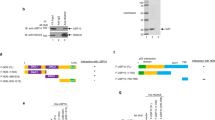
Cisplatin-based chemotherapy is the main treatment option for advanced or metastatic esophageal squamous cell carcinoma (ESCC). However, most ESCC patients develop drug resistance within 2 years after receiving cisplatin chemotherapy. Ubiquitin-specific protease 10 (USP10) is abnormally expressed in a variety of cancers, but the mechanistic roles of USP10 in ESCC are still obscure. Here, the effects of USP10 on the migration and cisplatin resistance of ESCC in vivo and in vitro and the underlying mechanisms have been investigated by bioinformatics analysis, RT-PCR, western blotting, immunoprecipitation, immunohistochemistry, cell migration and MTS cell proliferation assays, deubiquitination assay, and mouse tail vein injection model. USP10 was significantly up-regulated in ESCC tissues compared with adjacent normal tissues in both public databases and clinical samples and was closely associated with overall survival. Subsequent results revealed that USP10 contributed to the migration and cisplatin resistance of ESCC cells, while knocking down USP10 in cisplatin-resistant cells exhibited opposite effects in vitro and in vivo. Further Co-IP experiments showed that integrin β1 and YAP might be targets for USP10 deubiquitination. Moreover, deficiency of USP10 significantly inhibited the migrative and chemo-resistant abilities of ESCC cells, which could be majorly reversed by integrin β1 or YAP reconstitution. Altogether, USP10 was required for migration and cisplatin resistance in ESCC through deubiquinating and stabilizing integrin β1/YAP, highlighting that inhibition of USP10 may be a potential therapeutic strategy for ESCC.





Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- AML:
-
Acute myeloid leukemia
- DUBs:
-
Deubiquitinating enzyme
- HEEC:
-
Human esophageal epithelial cells
- USP10:
-
Ubiquitin-specific protease 10
- YAP:
-
Yes-associated protein
References
Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–73. https://doi.org/10.1053/j.gastro.2017.08.023.
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):51–6. https://doi.org/10.1093/annonc/mdt342.
Liu Y, Ren Z, Yuan L, Xu S, Yao Z, Qiao L, Li K. Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer. Am J Cancer Res. 2016;6:2345–50.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53. https://doi.org/10.1038/nm.3739.
Salas-Lloret D, González-Prieto R. Insights in post-translational modifications: ubiquitin and SUMO. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23063281].
Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–86. https://doi.org/10.1016/j.cell.2005.11.007.
Li Y, Reverter D. Molecular mechanisms of DUBs regulation in signaling and disease. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms22030986].
Sharma A, Alswillah T, Singh K, Chatterjee P, Willard B, Venere M, Summers MK, Almasan A. USP14 regulates DNA damage repair by targeting RNF168-dependent ubiquitination. Autophagy. 2018;14:1976–90. https://doi.org/10.1080/15548627.2018.1496877.
Xu G, Yang Z, Ding Y, Liu Y, Zhang L, Wang B, Tang M, Jing T, Jiao K, Xu X, et al. The deubiquitinase USP16 functions as an oncogenic factor in K-RAS-driven lung tumorigenesis. Oncogene. 2021;40:5482–94. https://doi.org/10.1038/s41388-021-01964-6.
Li X, Song N, Liu L, Liu X, Ding X, Song X, Yang S, Shan L, Zhou X, Su D, et al. USP9X regulates centrosome duplication and promotes breast carcinogenesis. Nat Commun. 2017;8:14866. https://doi.org/10.1038/ncomms14866.
Soncini C, Berdo I, Draetta G. Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease. Oncogene. 2001;20:3869–79. https://doi.org/10.1038/sj.onc.1204553.
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–96. https://doi.org/10.1016/j.cell.2009.12.032.
Jochemsen AG, Shiloh Y. USP10: friend and foe. Cell. 2010;140:308–10. https://doi.org/10.1016/j.cell.2010.01.034.
Bhattacharya U, Neizer-Ashun F, Mukherjee P, Bhattacharya R. When the chains do not break: the role of USP10 in physiology and pathology. Cell Death Dis. 2020;11:1033. https://doi.org/10.1038/s41419-020-03246-7.
Weisberg EL, Schauer NJ, Yang J, Lamberto I, Doherty L, Bhatt S, Nonami A, Meng C, Letai A, Wright R, et al. Inhibition of USP10 induces degradation of oncogenic FLT3. Nat Chem Biol. 2017;13:1207–15. https://doi.org/10.1038/nchembio.2486.
Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, Reynaert H, Halder G, van Grunsven LA. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679–88. https://doi.org/10.1016/j.jhep.2015.04.011.
Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. https://doi.org/10.1101/gad.274027.115.
Song J, Wang T, Chi X, Wei X, Xu S, Yu M, He H, Ma J, Li X, Du J, et al. Kindlin-2 inhibits the hippo signaling pathway by promoting degradation of MOB1. Cell Rep. 2019;29:3664-77.e3665. https://doi.org/10.1016/j.celrep.2019.11.035.
Ma S, Meng Z, Chen R, Guan KL. The hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. https://doi.org/10.1146/annurev-biochem-013118-111829.
Kofler M, Speight P, Little D, Di Ciano-Oliveira C, Szászi K, Kapus A. Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nat Commun. 2018;9:4966. https://doi.org/10.1038/s41467-018-07450-0.
Zhang J, Zhou Y, Tang PMK, Cheng ASL, Yu J, To KF, Kang W. Mechanotransduction and cytoskeleton remodeling shaping YAP1 in gastric tumorigenesis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20071576].
Sabra H, Brunner M, Mandati V, Wehrle-Haller B, Lallemand D, Ribba AS, Chevalier G, Guardiola P, Block MR, Bouvard D. β1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. J Biol Chem. 2017;292:19179–97. https://doi.org/10.1074/jbc.M117.808063.
Hou S, Jin W, Xiao W, Deng B, Wu D, Zhi J, Wu K, Cao X, Chen S, Ding Y, et al. Integrin α5 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Am J Cancer Res. 2019;9:2774–88.
Hou S, Hao X, Li J, Weng S, Wang J, Zhao T, Li W, Hu X, Deng B, Gu J, et al. TM4SF1 promotes esophageal squamous cell carcinoma metastasis by interacting with integrin α6. Cell Death Dis. 2022;13:609. https://doi.org/10.1038/s41419-022-05067-2.
Hou S, Hang Q, Isaji T, Lu J, Fukuda T, Gu J. Importance of membrane-proximal N-glycosylation on integrin β1 in its activation and complex formation. FASEB J. 2016;30:4120–31. https://doi.org/10.1096/fj.201600665R.
Hang Q, Isaji T, Hou S, Im S, Fukuda T, Gu J. Integrin α5 suppresses the phosphorylation of epidermal growth factor receptor and its cellular signaling of cell proliferation via N-glycosylation. J Biol Chem. 2015;290:29345–60. https://doi.org/10.1074/jbc.M115.682229.
Hang Q, Zeng L, Wang L, Nie L, Yao F, Teng H, Deng Y, Yap S, Sun Y, Frank SJ, et al. Non-canonical function of DGCR8 in DNA double-strand break repair signaling and tumor radioresistance. Nat Commun. 2021;12:4033. https://doi.org/10.1038/s41467-021-24298-z.
You BH, Yoon JH, Kang H, Lee EK, Lee SK, Nam JW. HERES, a lncRNA that regulates canonical and noncanonical Wnt signaling pathways via interaction with EZH2. Proc Natl Acad Sci U S A. 2019;116:24620–9. https://doi.org/10.1073/pnas.1912126116.
Chen SW, Zhou HF, Zhang HJ, He RQ, Huang ZG, Dang YW, Yang X, Liu J, Fu ZW, Mo JX, et al. The clinical significance and potential molecular mechanism of PTTG1 in Esophageal squamous cell carcinoma. Front Genet. 2020;11: 583085. https://doi.org/10.3389/fgene.2020.583085.
Wang W, Wei C, Li P, Wang L, Li W, Chen K, Zhang J, Zhang W, Jiang G. Integrative analysis of mRNA and lncRNA profiles identified pathogenetic lncRNAs in esophageal squamous cell carcinoma. Gene. 2018;661:169–75. https://doi.org/10.1016/j.gene.2018.03.066.
Zhu H, Yan F, Yuan T, Qian M, Zhou T, Dai X, Cao J, Ying M, Dong X, He Q, et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res. 2020;80:2204–16. https://doi.org/10.1158/0008-5472.can-19-2388.
Takayama KI, Suzuki T, Fujimura T, Takahashi S, Inoue S. Association of USP10 with G3BP2 inhibits p53 signaling and contributes to poor outcome in prostate cancer. Mol Cancer Res. 2018;16:846–56. https://doi.org/10.1158/1541-7786.mcr-17-0471.
Chen Q, Hang Y, Zhang T, Tan L, Li S, Jin Y. USP10 promotes proliferation and migration and inhibits apoptosis of endometrial stromal cells in endometriosis through activating the Raf-1/MEK/ERK pathway. Am J Physiol Cell Physiol. 2018;315:C863-c872. https://doi.org/10.1152/ajpcell.00272.2018.
Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. https://doi.org/10.1016/j.ccell.2016.05.005.
Yuan T, Chen Z, Yan F, Qian M, Luo H, Ye S, Cao J, Ying M, Dai X, Gai R, et al. Deubiquitinating enzyme USP10 promotes hepatocellular carcinoma metastasis through deubiquitinating and stabilizing Smad4 protein. Mol Oncol. 2020;14:197–210. https://doi.org/10.1002/1878-0261.12596.
Sun T, Xu YJ, Jiang SY, Xu Z, Cao BY, Sethi G, Zeng YY, Kong Y, Mao XL. Suppression of the USP10/CCND1 axis induces glioblastoma cell apoptosis. Acta Pharmacol Sin. 2021;42:1338–46. https://doi.org/10.1038/s41401-020-00551-x.
Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5:1639–49. https://doi.org/10.1016/j.celrep.2013.11.029.
Kim K, Huh T, Park Y, Koo DH, Kim H, Hwang I, Choi CH, Yi JM, Chung JY. Prognostic significance of USP10 and p14ARF expression in patients with colorectal cancer. Pathol Res Pract. 2020;216: 152988. https://doi.org/10.1016/j.prp.2020.152988.
Sun J, Li T, Zhao Y, Huang L, Sun H, Wu H, Jiang X. USP10 inhibits lung cancer cell growth and invasion by upregulating PTEN. Mol Cell Biochem. 2018;441:1–7. https://doi.org/10.1007/s11010-017-3170-2.
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS, Yang GF, Wang P, Fu GH. Prognostic significance of USP10 as a tumor-associated marker in gastric carcinoma. Tumour Biol. 2014;35:3845–53. https://doi.org/10.1007/s13277-013-1509-1.
Nakajima M, Kato H. Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother. 2013;14:1345–54. https://doi.org/10.1517/14656566.2013.801454.
Zeng Z, Li D, Yu T, Huang Y, Yan H, Gu L, Yuan J. Association and clinical implication of the USP10 and MSH2 proteins in non-small cell lung cancer. Oncol Lett. 2019;17:1128–38. https://doi.org/10.3892/ol.2018.9702.
Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–34. https://doi.org/10.1016/j.cell.2011.08.037.
Guo J, Zhang J, Liang L, Liu N, Qi M, Zhao S, Su J, Liu J, Peng C, Chen X, et al. Potent USP10/13 antagonist spautin-1 suppresses melanoma growth via ROS-mediated DNA damage and exhibits synergy with cisplatin. J Cell Mol Med. 2020;24:4324–40. https://doi.org/10.1111/jcmm.15093.
Liao Y, Guo Z, Xia X, Liu Y, Huang C, Jiang L, Wang X, Liu J, Huang H. Inhibition of EGFR signaling with Spautin-1 represents a novel therapeutics for prostate cancer. J Exp Clin Cancer Res. 2019;38:157. https://doi.org/10.1186/s13046-019-1165-4.
Fan YH, Cheng J, Vasudevan SA, Dou J, Zhang H, Patel RH, Ma IT, Rojas Y, Zhao Y, Yu Y, et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 2013;4: e867. https://doi.org/10.1038/cddis.2013.400.
Mooring M, Fowl BH, Lum SZC, Liu Y, Yao K, Softic S, Kirchner R, Bernstein A, Singhi AD, Jay DG, et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis. Hepatology. 2020;71:1813–30. https://doi.org/10.1002/hep.30928.
Guan H, Shang G, Cui Y, Liu J, Sun X, Cao W, Wang Y, Li Y. Long noncoding RNA APTR contributes to osteosarcoma progression through repression of miR-132-3p and upregulation of yes-associated protein 1. J Cell Physiol. 2019;234:8998–9007. https://doi.org/10.1002/jcp.27572.
Fan YP, Liao JZ, Lu YQ, Tian DA, Ye F, Zhao PX, Xiang GY, Tang WX, He XX. MiR-375 and doxorubicin co-delivered by liposomes for combination therapy of hepatocellular carcinoma. Mol Ther Nucleic acids. 2017;7:181–9. https://doi.org/10.1016/j.omtn.2017.03.010.
Qadir J, Riaz SK, Taj K, Sattar N, Sahar NE, Khan JS, Kayani MA, Haq F, Arshad Malik MF. Increased YAP1 expression is significantly associated with breast cancer progression, metastasis and poor survival. Future Oncol. 2021;17:2725–34. https://doi.org/10.2217/fon-2020-1080.
Zhang Z, Du J, Wang S, Shao L, Jin K, Li F, Wei B, Ding W, Fu P, van Dam H, et al. OTUB2 promotes cancer metastasis via hippo-independent activation of YAP and TAZ. Mol Cell. 2019;73:7-21.e27. https://doi.org/10.1016/j.molcel.2018.10.030.
Yi X, Deng X, Zhao Y, Deng B, Deng J, Fan H, Du Y, Hao L. Ubiquitin-like protein FAT10 promotes osteosarcoma growth by modifying the ubiquitination and degradation of YAP1. Exp Cell Res. 2020;387: 111804. https://doi.org/10.1016/j.yexcr.2019.111804.
Gao Y, Zhang X, Xiao L, Zhai C, Yi T, Wang G, Wang E, Ji X, Hu L, Shen G, et al. Usp10 modulates the hippo pathway by deubiquitinating and stabilizing the transcriptional Coactivator Yorkie. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20236013].
Qiu Y, Huang D, Sheng Y, Huang J, Li N, Zhang S, Hong Z, Yin X, Yan J. Deubiquitinating enzyme USP46 suppresses the progression of hepatocellular carcinoma by stabilizing MST1. Exp Cell Res. 2021;405: 112646. https://doi.org/10.1016/j.yexcr.2021.112646.
Kim JY, Lee DM, Woo HG, Kim KD, Lee HJ, Kwon YJ, Choi KS. RNAi screening-based identification of USP10 as a novel regulator of paraptosis. Sci Rep. 2019;9:4909. https://doi.org/10.1038/s41598-019-40982-z.
Funding
This study is partly supported by grants from the National Natural Science Foundation of China (Grant No. 32201047 to Q.H.), the Natural Science Foundation of Jiangsu Province (Grant No. BK20221373 to S.H.), the key project of Jiangsu Provincial Health Commission (Grant No. ZD2021038 to B.D.), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No. SCJX23_2033 to C.L.).
Author information
Authors and Affiliations
Contributions
SH and TZ: contributed to conceptualization, methodology, writing, and original draft preparation. CL and WL: contributed to investigation. BD and HH: contributed to validation. QH: contributed to supervision and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Affiliated Hospital of Yangzhou University. The guidelines for the care and use of animals were approved by the Medicine Animal Welfare Committee of Yangzhou University.
Consent for publication
The authors declared that the final version of the manuscript had been reviewed, approved, and consented to for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, S., Zhao, T., Deng, B. et al. USP10 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Med Oncol 41, 33 (2024). https://doi.org/10.1007/s12032-023-02272-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02272-7