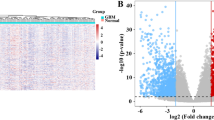
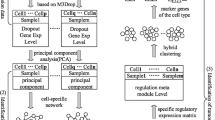
Abstract
Saffron crocus is a herbal medicine of traditional Tibetan medicine (TTM). Saffron extract has been indicated to inhibit tumor cell growth and promote tumor cell apoptosis in a variety of cancers, including glioma, but the specific mechanism is not clear. To study the possible mechanism of saffron action on glioma, network pharmacology and bioinformatics analysis methods were used in this study. We used the online database to obtain the active ingredients of saffron and their targets. Glioma-related targets were also acquired from online database. We intersected drug targets with glioma-related targets and conducted PPI network analysis to obtain network core genes. Then, we obtained RNA-seq data from The Cancer Genome Atlas (TCGA) database for glioma patients. Through different expression analysis and lasso regression, further screening of core genes in the network was conducted, and a prognostic model was established. The sample was divided into two groups with high and low risk using this model. The RNA-seq data from the Chinese Glioma Genome Atlas (CGGA) database were used to further validate our prediction model. Then, we explored the difference in pathways enrichment between high-risk patients and low-risk patients and calculated the difference in immune microenvironment between the two groups. Finally, we used scRNA-seq data in the CGGA database to analyze the cell types in which the model gene is mainly enriched and predicted the cell types which saffron effected on.










Similar content being viewed by others
Data availability
All data involved in this study were downloaded from public databases such as TCGA and CGGA. The patients involved in the database have obtained ethical approval. Users can download relevant data for free for research and publish relevant articles. Our study is based on open source data, so there are no ethical issues and other conflict of interest.
Abbreviations
- GBM:
-
Glioblastoma multiforme
- TCGA:
-
The Cancer Genome Atlas
- CGGA:
-
Chinese Glioma Genome Atlas
- PCA:
-
Principal component analysis
- TSNE:
-
T-distributed stochastic neighbor embedding
- UMAP:
-
Uniform manifold approximation and approximation projection
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- GSEA:
-
Gene set enrichment analysis
- TIME:
-
Tumor immune microenvironment
- PC:
-
Principal components
- BE:
-
Binding energy
- TTM:
-
Traditional Tibetan medicine
- TCMSP:
-
Traditional Chinese Medicine Systems Pharmacology Database
- OMIM:
-
Online Mendelian Inheritance in Man
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GBM:
-
Glioblastoma
- OB:
-
Oral bioavailability
- DL:
-
Drug-likeness
- PPI Network:
-
Protein–protein interaction network
- PDB:
-
Protein data bank
- BP:
-
Biological process
- CC:
-
Cellular component
- MF:
-
Molecular function
References
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. https://doi.org/10.1093/neuonc/nou087.
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–86. https://doi.org/10.1038/s41571-020-00447-z.
Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70:412–45. https://doi.org/10.1124/pr.117.014944.
Sousa-Pimenta M, Estevinho LM, Szopa A, Basit M, Khan K, Armaghan M, et al. Chemotherapeutic properties and side-effects associated with the clinical practice of terpene alkaloids: paclitaxel, docetaxel, and cabazitaxel. Front Pharmacol. 2023;14:1157306. https://doi.org/10.3389/fphar.2023.1157306.
Kwon YJ, Seo EB, Kim SK, Lee HS, Lee H, Jang YA, et al. Pharmacological anti-tumor effects of natural Chamaecyparis obtusa (siebold & zucc.) endl. Leaf extracts on breast cancer. J Ethnopharmacol. 2023;313:116598. https://doi.org/10.1016/j.jep.2023.116598.
Cordier W, Steenkamp P, Steenkamp V. Cytostatic and cytotoxic effects of a hot water and methanol extract of Acokanthera oppositifolia in HepG2 hepatocarcinoma cells. J Ethnopharmacol. 2023;314:116617. https://doi.org/10.1016/j.jep.2023.116617.
Chang H, Hou J, Shao Y, Xu M, Weng X, Du Y, et al. Sanggenon C inhibits cell proliferation and induces apoptosis by regulating the MIB1/DAPK1 axis in glioblastoma. MedComm. 2023;4:e281. https://doi.org/10.1002/mco2.281.
Hasan Abdali M, Afshar S, Sedighi Pashaki A, Dastan D, Gholami MH, Mahmoudi R, et al. Investigating the effect of radiosensitizer for ursolic acid and kamolonol acetate on HCT-116 cell line. Bioorg Med Chem. 2020;28:115152. https://doi.org/10.1016/j.bmc.2019.115152.
Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25:315–23. https://doi.org/10.1002/ptr.3311.
Kim TW, Lee HG. 6-Shogaol overcomes gefitinib resistance via ER stress in ovarian cancer cells. Int J Mol Sci. 2023;24:2639. https://doi.org/10.3390/ijms24032639.
Kantapan J, Dechsupa N, Tippanya D, Nobnop W, Chitapanarux I. Gallotannin from Bouea macrophylla seed extract suppresses cancer stem-like cells and radiosensitizes head and neck cancer. Int J Mol Sci. 2021;22:9253. https://doi.org/10.3390/ijms22179253.
Bauer-Wu S, Lhundup T, Tidwell T, Lhadon T, Ozawa-de Silva C, Dolma J, et al. Tibetan medicine for cancer: an overview and review of case studies. Integr Cancer Ther. 2014;13:502–12. https://doi.org/10.1177/1534735414549624.
Tang C, Zhao CC, Yi H, Geng ZJ, Wu XY, Zhang Y, et al. Traditional Tibetan medicine in cancer therapy by targeting apoptosis pathways. Front Pharmacol. 2020;11:976. https://doi.org/10.3389/fphar.2020.00976.
Harrington M. Saffron offers protection from liver cancer. Lab Anim (NY). 2011;40:289. https://doi.org/10.1038/laban1011-289a.
Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–67. https://doi.org/10.1002/hep.24433.
Amin A, Farrukh A, Murali C, Soleimani A, Praz F, Graziani G, et al. Saffron and its major ingredients’ effect on colon cancer cells with mismatch repair deficiency and microsatellite instability. Molecules. 2021;26:3855. https://doi.org/10.3390/molecules26133855.
Ege B, Yumrutas O, Ege M, Pehlivan M, Bozgeyik I. Pharmacological properties and therapeutic potential of saffron (Crocus sativus L.) in osteosarcoma. J Pharm Pharmacol. 2020;72:56–67. https://doi.org/10.1111/jphp.13179.
Moradzadeh M, Kalani MR, Avan A. The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: a mini review. J Cell Biochem. 2019;120:4732–8. https://doi.org/10.1002/jcb.27525.
Bhandari PR. Crocus sativus L. (saffron) for cancer chemoprevention: a mini review. J Tradit Complement Med. 2015;5:81–7. https://doi.org/10.1016/j.jtcme.2014.10.009.
Lambrianidou A, Koutsougianni F, Papapostolou I, Dimas K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules. 2020;26:86. https://doi.org/10.3390/molecules26010086.
Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46:3443–7. https://doi.org/10.1016/j.fct.2008.08.018.
Naeimi M, Shafiee M, Kermanshahi F, Khorasanchi Z, Khazaei M, Ryzhikov M, et al. Saffron (Crocus sativus) in the treatment of gastrointestinal cancers: current findings and potential mechanisms of action. J Cell Biochem. 2019;120:16330–9. https://doi.org/10.1002/jcb.29126.
Das I, Das S, Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: a histopathological study. Acta Histochem. 2010;112:317–27. https://doi.org/10.1016/j.acthis.2009.02.003.
Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for prevention and treatment of cancers. Nutrients. 2016;8:495. https://doi.org/10.3390/nu8080495.
Nezamdoost Z, Saghebjoo M, Hoshyar R, Hedayati M, Keska A. High-intensity training and saffron: effects on breast cancer-related gene expression. Med Sci Sports Exerc. 2020;52:1470–6. https://doi.org/10.1249/MSS.0000000000002274.
Baba SA, Vahedi M, Ahmad I, Rajab BS, Babalghith AO, Irfan S, et al. Crocus sativus L. tepal extract induces apoptosis in human U87 glioblastoma cells. Biomed Res Int. 2022;2022:4740246. https://doi.org/10.1155/2022/4740246.
Giakoumettis D, Pourzitaki C, Vavilis T, Tsingotjidou A, Kyriakoudi A, Tsimidou M, et al. Crocus sativus L. causes a non apoptotic calpain dependent death in C6 rat glioma cells, exhibiting a synergistic effect with temozolomide. Nutr Cancer. 2019;71:491–507. https://doi.org/10.1080/01635581.2018.1506493.
Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt H. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43:136–50. https://doi.org/10.1016/j.tips.2021.11.004.
Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12:56–68. https://doi.org/10.1038/nrg2918.
Tang Y, Li M, Wang J, Pan Y, Wu FX. CytoNCA: a cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems. 2015;127:67–72. https://doi.org/10.1016/j.biosystems.2014.11.005.
Colapietro A, Mancini A, Vitale F, Martellucci S, Angelucci A, Llorens S, et al. Crocetin extracted from saffron shows antitumor effects in models of human glioblastoma. Int J Mol Sci. 2020;21:423. https://doi.org/10.3390/ijms21020423.
Chen S, Ma J, Yang L, Teng M, Lai ZQ, Chen X, et al. Anti-glioblastoma activity of kaempferol via programmed cell death induction: involvement of autophagy and pyroptosis. Front Bioeng Biotechnol. 2020;8:614419. https://doi.org/10.3389/fbioe.2020.614419.
Kim EJ, Choi CH, Park JY, Kang SK, Kim YK. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res. 2008;33:971–9. https://doi.org/10.1007/s11064-007-9416-8.
Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK, Zhao WJ. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem Int. 2015;80:60–71. https://doi.org/10.1016/j.neuint.2014.12.001.
Cai F, Zhang Y, Li J, Huang S, Gao R. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. 2020. Biosci Rep. https://doi.org/10.1042/BSR20192826.
Li C, Li J, Li Y, Li L, Luo Y, Li J, et al. Isorhamnetin promotes MKN-45 gastric cancer cell apoptosis by inhibiting PI3K-mediated adaptive autophagy in a hypoxic environment. J Agric Food Chem. 2021;69:8130–43. https://doi.org/10.1021/acs.jafc.1c02620.
Wang JL, Quan Q, Ji R, Guo XY, Zhang JM, Li X, et al. Isorhamnetin suppresses PANC-1 pancreatic cancer cell proliferation through S phase arrest. Biomed Pharmacother. 2018;108:925–33. https://doi.org/10.1016/j.biopha.2018.09.105.
Hernandez Borrero LJ, El-Deiry WS. Tumor suppressor p53: biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 2021;1876:188556. https://doi.org/10.1016/j.bbcan.2021.188556.
Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol. 2021;11:760971. https://doi.org/10.3389/fonc.2021.760971.
Yadav P, Yadav R, Jain S, Vaidya A. Caspase-3: a primary target for natural and synthetic compounds for cancer therapy. Chem Biol Drug Des. 2021;98:144–65. https://doi.org/10.1111/cbdd.13860.
Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, Metabolism, and cancer. Cancer Discov. 2015;5:1024–39. https://doi.org/10.1158/2159-8290.CD-15-0507.
Li Y, Yu Y, Zhang Y, Zhou Y, Li C, Zhu J, et al. MAFIP is a tumor suppressor in cervical cancer that inhibits activation of the nuclear factor-kappa B pathway. Cancer Sci. 2011;102:2043–50. https://doi.org/10.1111/j.1349-7006.2011.02061.x.
Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–37. https://doi.org/10.1056/NEJMoa1006312.
Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. https://doi.org/10.1038/nri910.
Pavitra E, Kancharla J, Gupta VK, Prasad K, Sung JY, Kim J, et al. The role of NF-kappaB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed Pharmacother. 2023;163:114822. https://doi.org/10.1016/j.biopha.2023.114822.
Xiao L, Lan X, Shi X, Zhao K, Wang D, Wang X, et al. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell Death Dis. 2017;8:e2803. https://doi.org/10.1038/cddis.2017.210.
Mortezaee K, Najafi M, Farhood B, Ahmadi A, Shabeeb D, Musa AE. NF-kappaB targeting for overcoming tumor resistance and normal tissues toxicity. J Cell Physiol. 2019;234:17187–204. https://doi.org/10.1002/jcp.28504.
Funding
This work was supported by the Department of Science and Technology of Shandong Province (2020CXGC010903 and ZR2019ZD33), the Clinical Research Center of Shandong University (2020SDUCRCB002), and Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022003A).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Man, D., Zhao, P. et al. Identification of the therapeutic mechanism of the saffron crocus on glioma through network pharmacology and bioinformatics analysis. Med Oncol 40, 296 (2023). https://doi.org/10.1007/s12032-023-02142-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02142-2