Abstract
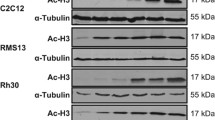
SMARCB1/INI1 deficiency is seen in several malignant tumors including malignant rhabdoid tumor (MRT), a highly aggressive pediatric malignancy. Loss of SMARCB1/INI1 function alters diverse oncogenic cellular signals, making it difficult to discover effective targeting therapy. By utilizing an in vitro drug screening system, effective therapeutic agents against SMARCB1/INI1-deficient tumors were explored in this study. In the in vitro drug sensitivity test, 80 agents with various actions were screened for their cytotoxicity in a panel of five SMARCB1/INI1-deficient tumor cell lines. The combination effect was screened based on the Bliss independent model. The growth-inhibitory effect was determined in both the conventional two-dimensional culture and the collagen-embedded three-dimensional culture system. Survivin expression after agent exposure was determined by Western blot analysis. All five cell lines were found to be sensitive to YM155, a selective survivin inhibitor. In the drug combination screening, YM155 showed additive to synergistic effects with various agents including chrysin. Chrysin enhanced YM155-induced apoptosis, but not mitochondrial depolarization upon exposure of SMARCB1/INI1-deficient tumor cells to the two agents for 6 h. YM155 and chrysin synergistically suppressed survivin expression, especially in TTN45 cells in which such suppression was observed as early as 6 h after exposure to the two agents. Survivin is suggested to be a therapeutic target in MRT and other SMARCB1/INI1-deficient tumors. Chrysin, a flavone that is widely distributed in plants, cooperatively suppressed survivin expression and enhanced the cytotoxicity of YM155.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Morgan KM, Siow VS, Strotmeyer S, Gow KW, Malek MM. Characteristics and outcomes in pediatric non-central nervous system malignant rhabdoid tumors: a report from the National Cancer Database. Ann Surg Oncol. 2022;29:671–8. https://doi.org/10.1245/s10434-021-10370-x.
Enault M, Minard-Colin V, Corradini N, Leverger G, Thebaud E, Rome A, Proust S, Marie-Cardine A, Defachelles AS, Sarnacki S, Philippe-Chomette P, Delattre O, Masliah-Planchon J, Lacour B, Ferrari A, Brennan B, Orbach D, Bourdeaut F. Extracranial rhabdoid tumours: results of a SFCE series of patients treated with a dose compression strategy according to European Paediatric Soft Tissue Sarcoma Study Group recommendations. Eur J Cancer. 2022;161:64–78. https://doi.org/10.1016/j.ejca.2021.10.025.
Brennan B, De Salvo GL, Orbach D, De Paoli A, Kelsey A, Mudry P, Francotte N, Van Noesel M, Bisogno G, Casanova M, Ferrari A. Outcome of extracranial malignant rhabdoid tumours in children registered in the European Paediatric Soft Tissue Sarcoma Study Group Non-Rhabdomyosarcoma Soft Tissue Sarcoma 2005 Study-EpSSG NRSTS 2005. Eur J Cancer. 2016;60:69–82. https://doi.org/10.1016/j.ejca.2016.02.027.
Margol AS, Judkins AR. Pathology and diagnosis of SMARCB1-deficient tumors. Cancer Genet. 2014;207:358–64. https://doi.org/10.1016/j.cancergen.2014.07.004.
Modena P, Lualdi E, Facchinetti F, Galli L, Teixeira MR, Pilotti S, Sozzi G. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012–9. https://doi.org/10.1158/0008-5472.CAN-04-3050.
Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108:547–52. https://doi.org/10.1111/cas.13173.
Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207:365–72. https://doi.org/10.1016/j.cancergen.2014.04.004.
Preusser M, Wolfsberger S, Czech T, Slavc I, Budka H, Hainfellner JA. Survivin expression in intracranial ependymomas and its correlation with tumor cell proliferation and patient outcome. Am J Clin Pathol. 2005;124:543–9. https://doi.org/10.1309/PP2G5GAAFKV82DTG.
Karpinsky G, Krawczyk MA, Izycka-Swieszewska E, Fatyga A, Budka A, Balwierz W, Sobol G, Zalewska-Szewczyk B, Rychlowska-Pruszynska M, Klepacka T, Dembowska-Baginska B, Kazanowska B, Gabrych A, Bien E. Tumor expression of survivin, p53, cyclin D1, osteopontin and fibronectin in predicting the response to neo-adjuvant chemotherapy in children with advanced malignant peripheral nerve sheath tumor. J Cancer Res Clin Oncol. 2018;144:519–29. https://doi.org/10.1007/s00432-018-2580-1.
Uehara S, Oue T, Kawatsu M, Nara K, Fukuzawa M. Increased expression of survivin in hepatoblastoma after chemotherapy. Eur J Pediatr Surg. 2013;23:400–4. https://doi.org/10.1055/s-0033-1333637.
Rafatmanesh A, Behjati M, Mobasseri N, Sarvizadeh M, Mazoochi T, Karimian M. The survivin molecule as a double-edged sword in cellular physiologic and pathologic conditions and its role as a potential biomarker and therapeutic target in cancer. J Cell Physiol. 2020;235:725–44. https://doi.org/10.1002/jcp.29027.
Takamizawa S, Scott D, Wen J, Grundy P, Bishop W, Kimura K, Sandler A. The survivin:fas ratio in pediatric renal tumors. J Pediatr Surg. 2001;36:37–42. https://doi.org/10.1053/jpsu.2001.20000.
Takita J, Chen Y, Kato M, Ohki K, Sato Y, Ohta S, Sugita K, Nishimura R, Hoshino N, Seki M, Sanada M, Oka A, Hayashi Y, Ogawa S. Genome-wide approach to identify second gene targets for malignant rhabdoid tumors using high-density oligonucleotide microarrays. Cancer Sci. 2014;105:258–64. https://doi.org/10.1111/cas.12352.
Goto H, Takahashi H, Funabiki T, Ikuta K, Sasaki H, Nagashima Y. Brief report: neural differentiation of a novel cell line, YCUS-5, established from proximal-type epithelioid sarcoma of a child. Med Pediatr Oncol. 1999;33:137–8. https://doi.org/10.1002/(sici)1096-911x(199908)33:2%3c137::aid-mpo18%3e3.0.co;2-n.
Goto H, Yoshino Y, Ito M, Nagai J, Kumamoto T, Inukai T, Sakurai Y, Miyagawa N, Keino D, Yokosuka T, Iwasaki F, Hamanoue S, Shiomi M, Goto S. Aurora B kinase as a therapeutic target in acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;85:773–83. https://doi.org/10.1007/s00280-020-04045-9.
Szulkin A, Otvös R, Hillerdal CO, Celep A, Yousef-Fadhel E, Skribek H, Hjerpe A, Székely L, Dobra K. Characterization and drug sensitivity profiling of primary malignant mesothelioma cells from pleural effusions. BMC Cancer. 2014;14:709. https://doi.org/10.1186/1471-2407-14-709.
Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3: e00149. https://doi.org/10.1002/prp2.149.
Zoetemelk M, Rausch M, Colin DJ, Dormond O, Nowak-Sliwinska P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep. 2019;9:7103. https://doi.org/10.1038/s41598-019-42836-0.
Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschläger M, Kenner L, Pfeiffer D, Krupitza G, Dolznig H. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017;130:203–18. https://doi.org/10.1242/jcs.188102.
Rubie H, Geoerger B, Frappaz D, Schmitt A, Leblond P, Ndiaye A, Aerts I, Le Deley MC, Gentet JC, Paci A, Chastagner P, Dias N, Djafari L, Pasquet M, Chatelut E, Landman-Parker J, Corradini N, Vassal G. Phase I study of topotecan in combination with temozolomide (TOTEM) in relapsed or refractory paediatric solid tumours. Eur J Cancer. 2010;46:2763–70. https://doi.org/10.1016/j.ejca.2010.05.004.
Hawkins DS, Bradfield S, Whitlock JA, Krailo M, Franklin J, Blaney SM, Adamson PC, Reaman G. Topotecan by 21-day continuous infusion in children with relapsed or refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2006;47:790–4. https://doi.org/10.1002/pbc.20739.
Goto H, Yanagimachi M, Goto S, Takeuchi M, Kato H, Yokosuka T, Kajiwara R, Yokota S. Methylated chrysin reduced cell proliferation, but antagonized cytotoxicity of other anticancer drugs in acute lymphoblastic leukemia. Anticancer Drugs. 2012;23:417–25. https://doi.org/10.1097/CAD.0b013e32834fb731.
Minoda M, Kawamoto T, Ueha T, Kamata E, Morishita M, Harada R, Toda M, Onishi Y, Hara H, Kurosaka M, Akisue T. Antitumor effect of YM155, a novel small-molecule survivin suppressant, via mitochondrial apoptosis in human MFH/UPS. Int J Oncol. 2015;47:891–9. https://doi.org/10.3892/ijo.2015.3077.
Oda Y, Tsuneyoshi M. Extrarenal rhabdoid tumors of soft tissue: clinicopathological and molecular genetic review and distinction from other soft-tissue sarcomas with rhabdoid features. Pathol Int. 2006;56:287–95. https://doi.org/10.1111/j.1440-1827.2006.01962.x.
Kurmasheva RT, Erickson SW, Earley E, Smith MA, Houghton PJ. In vivo evaluation of the EZH2 inhibitor (EPZ011989) alone or in combination with standard of care cytotoxic agents against pediatric malignant rhabdoid tumor preclinical models—a report from the Pediatric Preclinical Testing Consortium. Pediatr Blood Cancer. 2021;68: e28772. https://doi.org/10.1002/pbc.28772.
Shimizu T, Nishio K, Sakai K, Okamoto I, Okamoto K, Takeda M, Morishita M, Nakagawa K. Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2020;86:211–9. https://doi.org/10.1007/s00280-020-04112-1.
Papadopoulos KP, Lopez-Jimenez J, Smith SE, Steinberg J, Keating A, Sasse C, Jie F, Thyss A. A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk Lymphoma. 2016;57:1848–55. https://doi.org/10.3109/10428194.2015.1113275.
Clemens MR, Gladkov OA, Gartner E, Vladimirov V, Crown J, Steinberg J, Jie F, Keating A. Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2015;149:171–9. https://doi.org/10.1007/s10549-014-3238-6.
Kudchadkar R, Ernst S, Chmielowski B, Redman BG, Steinberg J, Keating A, Jie F, Chen C, Gonzalez R, Weber J. A phase 2, multicenter, open-label study of sepantronium bromide (YM155) plus docetaxel in patients with stage III (unresecn) or stage IV melanoma. Cancer Med. 2015;4:643–50. https://doi.org/10.1002/cam4.363.
Tolcher AW, Quinn DI, Ferrari A, Ahmann F, Giaccone G, Drake T, Keating A, de Bono JS. A phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer. Ann Oncol. 2012;23:968–73. https://doi.org/10.1093/annonc/mdr353.
Mondal A, Jia D, Bhatt V, Akel M, Roberge J, Guo JY, Langenfeld J. Ym155 localizes to the mitochondria leading to mitochondria dysfunction and activation of AMPK that inhibits BMP signaling in lung cancer cells. Sci Rep. 2022;12:13135. https://doi.org/10.1038/s41598-022-17446-y.
Peery R, Cui Q, Kyei-Baffour K, Josephraj S, Huang C, Dong Z, Dai M, Zhang JT, Liu JY. A novel survivin dimerization inhibitor without a labile hydrazone linker induces spontaneous apoptosis and synergizes with docetaxel in prostate cancer cells. Bioorg Med Chem. 2022;65: 116761. https://doi.org/10.1016/j.bmc.2022.116761.
Goto H, Kitagawa N, Sekiguchi H, Miyagi Y, Keino D, Sugiyama M, Sarashina T, Miyagawa N, Yokosuka T, Hamanoue S, Iwasaki F, Shiomi M, Goto S, Tanaka Y. The collagen gel droplet-embedded culture drug sensitivity test in relapsed hepatoblastoma. J Pediatr Hematol Oncol. 2017;39:395–401. https://doi.org/10.1097/MPH.0000000000000865.
Kii T, Sakuma K, Tanaka A. Optimal contact concentration of paclitaxel in the collagen gel droplet-embedded culture drug sensitivity test for human oral squamous cell carcinoma and evaluation of combination with cetuximab. Chemotherapy. 2021;65:147–57. https://doi.org/10.1159/000512542.
Kobayashi H. Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness. Recent Results Cancer Res. 2003;161:48–61. https://doi.org/10.1007/978-3-642-19022-3_5.
Garg A, Chaturvedi S. A comprehensive review on chrysin: emphasis on molecular targets, pharmacological actions and bio-pharmaceutical aspects. Curr Drug Targets. 2022;23:420–36. https://doi.org/10.2174/1389450122666210824141044.
Zhou L, Yang C, Zhong W, Wang Q, Zhang D, Zhang J, Xie S, Xu M. Chrysin induces autophagy-dependent ferroptosis to increase chemosensitivity to gemcitabine by targeting CBR1 in pancreatic cancer cells. Biochem Pharmacol. 2021;193: 114813. https://doi.org/10.1016/j.bcp.2021.114813.
Park W, Park S, Lim W, Song G. Chrysin disrupts intracellular homeostasis through mitochondria-mediated cell death in human choriocarcinoma cells. Biochem Biophys Res Commun. 2018;503:3155–61. https://doi.org/10.1016/j.bbrc.2018.08.109.
Ganai SA, Sheikh FA, Baba ZA. Plant flavone chrysin as an emerging histone deacetylase inhibitor for prosperous epigenetic-based anticancer therapy. Phytother Res. 2021;35:823–34. https://doi.org/10.1002/ptr.6869.
Pal-Bhadra M, Ramaiah MJ, Reddy TL, Krishnan A, Pushpavalli SN, Babu KS, Tiwari AK, Rao JM, Yadav JS, Bhadra U. Plant HDAC inhibitor chrysin arrest cell growth and induce p21WAF1 by altering chromatin of STAT response element in A375 cells. BMC Cancer. 2012;12:180. https://doi.org/10.1186/1471-2407-12-180.
Gao S, Siddiqui N, Etim I, Du T, Zhang Y, Liang D. Developing nutritional component chrysin as a therapeutic agent: bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed Pharmacother. 2021;142: 112080. https://doi.org/10.1016/j.biopha.2021.112080.
Funding
This work was supported by Kanagawa Children’s Medical Foundation; Kanagawa Prefectural Hospital Organization.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Cell lines were established and maintained by HG, JT, and YH. Material preparation, data collection, and analysis were performed by YY, MI, YT, and MY. The first draft of the manuscript was written by YY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest related to the presented study.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Kanagawa Children’s Medical Center (09/14/2017, No. 105-8).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12032_2022_1843_MOESM1_ESM.tif
Supplementary file1 (TIF 250 KB)—Supplemental Figure 1. In vitro drug sensitivity screening in a panel of SMARCB1/INI1-deficient cell lines. Cells were injected onto a 384-well plate, and after 1-day incubation, 80 agents belonging to several different classes at 4 serially diluted concentrations were loaded onto the wells. After 4 days’ incubation, cell viability in each well was measured using the CellTiter-Glo luminescent assay (Promega, Madison, WI, USA). Cell survival rates in agent-containing wells were expressed as ratios of live cell-derived luminescence compared to the mean luminescence value in 5 agent-free control wells. To compare agent sensitivity among the samples, the drug effect score (DES) was calculated for each agent as follows: DES = {(100 − % survival at 5–3 dilution)*ln(125) + (100 − % survival at 5–2 dilution)*ln(25) + (100 − % survival at 5–1 dilution)*ln(5) + (100 − % survival at no dilution)}/{ln(125) + ln(25) + ln(5) + 1}. A cell line was defined to be sensitive to the tested agent if its DES was larger than the reference DES (i.e., the mean DES when peripheral blood mononuclear cells from 5 healthy volunteers were tested in the same drug sensitivity test). In the heat map of Fig. 1, the number represents the DES of the tested agent at a particular concentration after subtracting the corresponding reference DES. A red box indicates high DES; the more intense the redness, the higher the DES. Topotecan (red arrow) and YM155 (red arrow) showed relatively high DES values in the 5 cell lines. The highest concentrations of agents used in the screening assay are shown in Supplemental Table 1.
12032_2022_1843_MOESM2_ESM.tif
Supplementary file2 (TIF 698 KB)—Supplemental Figure 2. Drug combination screening. The combination indexes between YM155 at 10 nM and agents with various modes of action at 4 serial dilutions are shown. A red box indicates that the combination index is less than 1.0, suggesting a synergistic effect.
12032_2022_1843_MOESM3_ESM.tif
Supplementary file3 (TIF 464 KB)—Supplemental Figure 3. Apoptotic change of nuclei after agent exposure. Cells were seeded onto a 24-well glass bottom plate with control medium, medium containing YM155 alone at 10 nM, medium containing chrysin alone at 4 μg/ml, or medium containing the combination of YM155 at 10 nM and chrysin at 4 μg/ml. After 48 h incubation, cellular nuclei were stained with NucBlue Live ReadyProbes Reagent (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Nuclear blebbing indicating apoptotic change was observed upon incubation with YM155, but was not evident upon incubation with chrysin alone. (Original magnification: ×200).
12032_2022_1843_MOESM4_ESM.tif
Supplementary file4 (TIF 823 KB)—Supplemental Figure 4. Loss of mitochondrial transmembrane potential after agent exposure. Fluorescent microscopic pictures after JC-1 staining are shown. See Figure Legend 4 for details. (Original magnification: ×100).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshino, Y., Goto, H., Ito, M. et al. YM155 and chrysin cooperatively suppress survivin expression in SMARCB1/INI1-deficient tumor cells. Med Oncol 39, 234 (2022). https://doi.org/10.1007/s12032-022-01843-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01843-4