Abstract
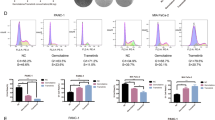
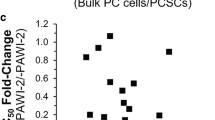
Pancreatic ductal adenocarcinoma (PDAC) is among the most deadly cancers. Since most patients develop resistance to conventional treatments, new approaches are in urgency. Valproic acid (VPA) was shown to induce apoptosis and reduce proliferation in PANC-1 cells. Wnt signaling pathway is known to be involved in apoptosis and PDAC onset. However, VPA-induced apoptosis and its impact on Wnt signaling in PDACs are not linked, yet. We aimed to calculate IC50 of VPA-induced PANC-1 cells by combined analyses of proliferation and apoptosis, while assessing its effect on Wnt signaling pathway. PANC-1 was induced with increased VPA doses and time points. Three independent proliferation and apoptosis assays were performed utilizing carboxyfluorescein succinimidyl ester and Annexin V/PI staining, respectively. Flow cytometry measurements were analyzed by CellQuest and NovoExpress. Taqman hydrolysis probes and SYBR Green PCR Mastermix were assessed in expression analyses of Wnt components utilizing 2−ΔΔCt method. Cell proliferation was inhibited by 50% at 2.5 mM VPA that evoked a significant apoptotic response. Among the screened Wnt components and target genes, only LEF1 exhibited significant four-fold upregulation at this concentration. In conclusion, cancer studies mostly utilize MTT or BrdU assays in estimating cell proliferation and calculating IC50 of drugs, which provided conflicting VPA dosages utilizing PANC-1 cells. Our novel combined approach enabled specific, accurate and reproducible IC50 calculation at single cell basis with no apparent effect on Wnt signaling components. Future studies are needed to clarify the role of LEF1 in this model.


Similar content being viewed by others
References
McGuire S, World Cancer Report. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2014. https://doi.org/10.3945/an.116.012211.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. https://doi.org/10.3322/caac.21492.
Gilardini Montani MS, Granato M, Santoni C, Del Porto P, Merendino N, D’Orazi G, et al. Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells. Cell Oncol (Dordr). 2017. https://doi.org/10.1007/s13402-017-0314-z.
Kleger A, Perkhofer L, Seufferlein T. Smarter drugs emerging in pancreatic cancer therapy. Ann Oncol. 2014. https://doi.org/10.1093/annonc/mdu013.
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002. https://doi.org/10.1038/nrg816.
Spange S, Wagner T, Heinzel T, Krämer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009. https://doi.org/10.1016/j.biocel.2008.08.027.
Blaheta RA, Cinatl J Jr. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002. https://doi.org/10.1002/med.10017.
Michaelis M, Michaelis UR, Fleming I, Suhan T, Cinatl J, Blaheta RA, Hoffmann K, Kotchetkov R, Busse R, Nau H, Cinatl J Jr. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004. https://doi.org/10.1124/mol.65.3.520.
Blaheta RA, Michaelis M, Driever PH, Cinatl J Jr. Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev. 2005. https://doi.org/10.1002/med.20027.
Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008. https://doi.org/10.1016/j.ctrv.2007.11.003.
Li Y, Liu T, Ivan C, Huang J, Shen DY, Kavanagh JJ, et al. Enhanced cytotoxic effects of combined valproic acid and the aurora kinase inhibitor VE465 on gynecologic cancer cells. Front Oncol. 2013. https://doi.org/10.3389/fonc.2013.00058.
Kostrouchová M, Kostrouch Z, Kostrouchová M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol (Praha). 2007;53:37–49.
Jamieson C, Sharma M, Henderson BR. Targeting the β-catenin nuclear transport pathway in cancer. Semin Cancer Biol. 2014. https://doi.org/10.1016/j.semcancer.2014.04.012.
Sarkar S, Mandal C, Sangwan R, Mandal C. Coupling G2/M arrest to the Wnt/β-catenin pathway restrains pancreatic adenocarcinoma. Endocr Relat Cancer. 2014. https://doi.org/10.1530/ERC-13-0315.
Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999. https://doi.org/10.1046/j.1440-1711.1999.00877.x.
Raz S, Sheban D, Gonen N, Stark M, Berman B, Assaraf YG. Severe hypoxia induces complete antifolate resistance in carcinoma cells due to cell cycle arrest. Cell Death Dis. 2014. https://doi.org/10.1038/cddis.2014.39.
Ahlen MT, Husebekk A, Killie MK, Skogen B, Stuge TB. T-cell responses associated with neonatal alloimmune thrombocytopenia: isolation of HPA-1a-specific, HLA-DRB3*0101-restricted CD4+ T cells. Blood. 2009. https://doi.org/10.1182/blood-2008-09-178475.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995. https://doi.org/10.1016/0022-1759(95)00072-i.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, et al. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019. https://doi.org/10.2174/1570159X17666181227165722.
Lichun S, David H. Anti-convulsant drug valproic acid in cancers and in combination anti-cancer therapeutics. Mod Chem Appl. 2014. https://doi.org/10.4172/2329-6798.1000118.
Münster P, Marchion D, Bicaku E, Schmitt M, Lee JH, DeConti R, et al. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncol. 2007. https://doi.org/10.1200/JCO.2006.08.6165.
Lehner B, Sandner B, Marschallinger J, Lehner C, Furtner T, Couillard-Despres S, et al. The dark side of BrdU in neural stem cell biology: detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011. https://doi.org/10.1007/s00441-011-1213-7.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. https://doi.org/10.1016/0022-1759(83)90303-4.
Han M, Li JF, Tan Q, Sun YY. Limitations of the use of MTT assay for screening in drug discovery. J Chin Pharmaceutical Sci. 2010. https://doi.org/10.5246/jcps.2010.03.027.
Lü L, Zhang L, Wai MS, Yew DT, Xu J. Exocytosis of MTT formazan could exacerbate cell injury. Toxicol In Vitro. 2012. https://doi.org/10.1016/j.tiv.2012.02.006.
Miyashita T, Miki K, Kamigaki T, Makino I, Tajima H, Nakanuma S, et al. Low-dose valproic acid with low-dose gemcitabine augments MHC class I-related chain A/B expression without inducing the release of soluble MHC class I-related chain A/B. Oncol Lett. 2017. https://doi.org/10.3892/ol.2017.6943.
Li H, Zhang Z, Gao C, Wu S, Duan Q, Wu H, et al. Combination chemotherapy of valproic acid (VPA) and gemcitabine regulates STAT3/Bmi1 pathway to differentially potentiate the motility of pancreatic cancer cells. Cell Biosci. 2019. https://doi.org/10.1186/s13578-019-0312-0.
Venkataramani V, Rossner C, Iffland L, Schweyer S, Tamboli IY, Walter J, et al. Histone deacetylase inhibitor valproic acid inhibits cancer cell proliferation via down-regulation of the alzheimer amyloid precursor protein. J Biol Chem. 2010. https://doi.org/10.1074/jbc.M109.057836.
Li XN, Shu Q, Su JM, Perlaky L, Blaney SM, Lau CC. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol Cancer Ther. 2005;4:1912–22.
Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012. https://doi.org/10.1038/nature11005.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012. https://doi.org/10.1038/nature11003.
Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008. https://doi.org/10.1053/j.gastro.2008.06.089.
Wang L, Heidt DG, Lee CJ, Yang H, Logsdon CD, Zhang L, et al. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009. https://doi.org/10.1016/j.ccr.2009.01.018.
Jesse S, Koenig A, Ellenrieder V, Menke A. Lef-1 isoforms regulate different target genes and reduce cellular adhesion. Int J Cancer. 2010. https://doi.org/10.1002/ijc.24802.
Santiago L, Daniels G, Wang D, Deng FM, Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7:1389–406.
Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T, et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007. https://doi.org/10.1371/journal.pone.0001155.
Acknowledgements
PANC-1 cell line was kindly provided by Thomas Linn, M.D. and Ali Osman Gurol, M.D Ph.D. in support of their joint project TÜBİTAK-INTENC Project No. 113S029.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University. Project No.: 32763.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing or conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ekici, Y., Yilmaz, A., Kucuksezer, U.C. et al. Combined evaluation of proliferation and apoptosis to calculate IC50 of VPA-induced PANC-1 cells and assessing its effect on the Wnt signaling pathway. Med Oncol 38, 109 (2021). https://doi.org/10.1007/s12032-021-01560-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01560-4