Abstract
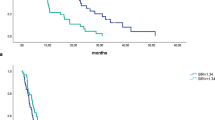
Inflammation is known to play an important role in the biology of metastatic pancreatic adenocarcinoma (mPDAC), and systemic inflammatory response (SIR) is reported to be closely related to outcome in mPDAC. The aim of the present paper is to assess the prognostic performance of SIR-related variables in patients with mPDAC receiving cytotoxic chemotherapy. Data of patients with mPDAC, who were registered in the database of the division of Medical Oncology of the G. Borea Hospital in Sanremo, were retrospectively collected. Three SIR-related variables were calculated. Univariate and multivariate analyses have been performed by Cox regression, and every SIR-related variable was added to the Cox model. After univariate analyses, four variables reported a significant relationship with overall survival (OS) and were included in a Cox model. Two of the three SIR-related variables were related with OS in the bivariate analyses, and maintained their independent prognostic effect when added to the previous Cox model in multivariate analysis. These variables were two scores, such as neutrophil-platelet score (NPS; HR 2.144, CIs 1.392–3.300) and tri-linear peripheric blood cell score (TRIS; HR 1.813, CIs 1.331–2.468). It appears that the second-generation inflammation-related variables are more effective in predicting prognosis of patients with mPDAC receiving chemotherapy.
Similar content being viewed by others
References
Malvezzi M, Cairoli G, Bertuccio P, et al. European cancer mortality predictiona for the year 2016 with focus on leukemias. Ann Oncol. 2016;27:725–31.
Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:18–48.
Goldstein D, Hassan El-Maraghi R, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer. Long-term suvival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells, in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation on the Th2 cytokine interlaukin-13. Cancer Immunol Immunother. 2011;60:1419–30.
Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobil Pancreat Dis Int. 2014;13:474–81.
Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2016;346:1234–8.
Tao L, Zhang L, Peng Y, et al. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma. Medicine. 2016;95:39.
Gao Y, Wang W-J, Zhi Q, et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget. 2017;8:88835–44.
Suzuki R, Takagi T, Hikichi T, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett. 2016;11:3441–5.
Xue P, Hang J, Huang W, et al. Validation of lymphocyte-to monocyte ratio as a prognostic factor in advanced pancreatic cancer: an East asian cohort study of 2 country. Pancreas. 2017;46:1011–7.
Martin HL, Ohara K, Kiberu A, Van Hagen T, Davidson A, Khattak MA. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J. 2014; 676 – 82.
Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels. Ann Surg. 2018. https://doi.org/10.1097/SLA.0000000000002660.
Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122:2158–67.
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–7.
Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC. The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS ONE. 2015;10:e0142159.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matriian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Papadoniou N, Kosmas C, Gennatas K, et al. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res. 2008;28:543–50.
Tao L, Yuan C, Ma Z, Jiang B, Xiu D. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: a population-based study. Cancer Manage Res. 2017;9:471–79.
Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a Surveillance Epidemiology and End Results database analysis. World J Gastroenterol. 2017;23:1872–80.
Zheng B, Ohuchida K, Yan Z, Okumura T, Ohtsuka T, Nakamura M. Primary recurrence in the lung is related to favorable prognosis in patients with pancreatic cancer and post-operative recurrence. World J Surg. 2017;41:2858–66.
Wangjam T, Zhang Z, Zhou XC, et al. Resected pancreatic ductal adenocarcinoma with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget. 2015;6:36903–10.
Downs-Canner S, Zenati M, Boone BA, et al. The indolent nature of pulmonary metastases from ductal adenocarcinoma of the pancreas. J Surg Oncol. 2015;112:80–5.
Tas F, Karabulut S, Ciftici R, et al. Serum levels of LDH, CEA, and CA19-9 have prognostic roles on survival in patients with metastatic pancreatic cancer receiving gemcitabine-based chemotherapy. Cancer Chemother Pharmacol. 2014;73:1163–71.
Haas M, Heinemann V, Kullmann F, et al. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol. 2013;139:681–9.
Tsavaris N, Kosmas C, Papadoniou N, et al. CEA and CA-19.9 serum tumor markers as prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. J Chemother. 2009;21:673–80.
Mundy-Bosse BL, Young GS, Bauer T, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4 + T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–79.
Annels NE, Shaw VE, Gabitass RF, et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:175–83.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep. 2015;5:11026.
Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–6.
Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–21.
Stevens L, Pathak S, Nunes QM, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HBP. 2015;17:285–91.
Miao C, Zhu S, Pan H, et al. Combined neutrophil-platelet score and hemoglobin level predict survival in esophageal squamous cell carcinoma patients treated with chemotadiotherapy. Oncotarget. 2017;8:87971–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Regione Liguria research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Colloca, G.A., Venturino, A. & Guarneri, D. Second-generation inflammation-related scores are more effective than systemic inflammation ratios in predicting prognosis of patients with unresectable or metastatic pancreatic cancer receiving cytotoxic chemotherapy. Med Oncol 35, 158 (2018). https://doi.org/10.1007/s12032-018-1219-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1219-z