Abstract
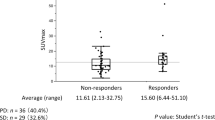
Recent advances in positron emission tomography with fluorine-18-fluorodeoxyglucose (FDG-PET) have facilitated not only the diagnosis and staging of lung cancer, but also the prediction of treatment outcome. The present study was designed to assess the usefulness of early FDG-PET examination for predicting subsequent tumor size reduction in response to molecular targeted agents in metastatic non-small cell lung cancer (NSCLC) with sensitive gene anomalies. I. In 29 targeted lesions of 10 NSCLC patients, changes in FDG uptake before and on day 7 after the initiation of molecular targeted therapy (gefitinib, n = 7; crizotinib, n = 3) were compared with subsequent radiographic tumor size reduction by RECIST. FDG uptake was evaluated as the maximum standardized uptake value (SUVmax) of each targeted lesion. SUVmax decreased in all lesions after therapy (mean SUVmax 8.3 ± 3.4 before to 3.7 ± 1.8 after therapy, p < 0.05). The % decrease in SUVmax of each lesion was significantly correlated with the % tumor size reduction (r = 0.44). In addition, the reduction rate of SUVmax in metastatic bone lesions after initiation of molecular targeted therapy was significantly lower than that in targeted organs (27.1 ± 27.5 vs. 51.2 ± 21.3%, respectively, p < 0.05). Early reduction in FDG-PET uptake after initiation of molecular targeted agents was able to predict subsequent tumor reduction in patients harboring EGFR-mutated or ALK-positive NSCLC. In addition, nontargeted bone metastasis may have different glucose metabolism after TKI treatment compared with other involved organs.



Similar content being viewed by others
References
Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T; North-East Japan Study Group. M. Maemondo, A. Inoue, K. Kobayashi, S. Sugawara, S. Oizumi, H. Isobe, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Ku GY, Haaland BA, de Lima Lopes G Jr. Gefitinib vs. chemotherapy as first-line therapy in advanced non-small cell lung cancer: meta-analysis of phase III trials. Lung Cancer. 2011;74:469–73.
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F, PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77.
Fukuhara T, Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Factors associated with a poor response to gefitinib in the NEJ002 study: smoking and the L858R mutation. Lung Cancer. 2015;88:181–6.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703.
Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 2008;99:929–35.
Park S, Holmes-Tisch AJ, Cho EY, Shim YM, Kim J, Kim HS, Lee J, Park YH, Ahn JS, Park K, Jänne PA, Ahn MJ. Discordance of molecular biomarkers associated with epidermal growth factor receptor pathway between primary tumors and lymph node metastasis in non-small cell lung cancer. J Thorac Oncol. 2009;4:809–15.
Gow CH, Chang YL, Hsu YC, Tsai MF, Wu CT, Yu CJ, Yang CH, Lee YC, Yang PC, Shih JY. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol. 2009;20:696–702.
Ushiki A, Koizumi T, Kobayashi N, Kanda S, Yasuo M, Yamamoto H, Kubo K, Aoyagi D, Nakayama J. Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn J Clin Oncol. 2009;39:267–70.
Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50(Suppl 1):31S–42S.
Paesmans M, Berghmans T, Dusart M, Garcia C, Hossein-Foucher C, Lafitte JJ, Mascaux C, Meert AP, Roelandts M, Scherpereel A, Terrones Munoz V, Sculier JP. European Lung Cancer Working Party, and on behalf of the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5:612–9.
Uehara H, Tsutani Y, Okumura S, Nakayama H, Adachi S, Yoshimura M, Miyata Y, Okada M. Prognostic role of positron emission tomography and high-resolution computed tomography in clinical stage IA lung adenocarcinoma. Ann Thorac Surg. 2013;96:1958–65.
Shang J, Ling X, Zhang L, Tang Y, Xiao Z, Cheng Y, Guo B, Gong J, Huang L, Xu H. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2016;43:1945–53.
Na II, Byun BH, Kang HJ, Cheon GJ, Koh JS, Kim CH, Choe DH, Ryoo BY, Lee JC, Lim SM, Yang SH. 18F-fluoro-2-deoxy-glucose uptake predicts clinical outcome in patients with gefitinib-treated non-small cell lung cancer. Clin Cancer Res. 2008;14:2036–41.
Takahashi R, Hirata H, Tachibana I, Shimosegawa E, Inoue A, Nagatomo I, Takeda Y, Kida H, Goya S, Kijima T, Yoshida M, Kumagai T, Kumanogoh A, Okumura M, Hatazawa J, Kawase I. Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res. 2012;18:220–8.
Sunaga N, Oriuchi N, Kaira K, Yanagitani N, Tomizawa Y, Hisada T, Ishizuka T, Endo K, Mori M. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59:203–10.
Su H, Bodenstein C, Dumont RA, Seimbille Y, Dubinett S, Phelps ME, Herschman H, Czernin J, Weber W. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:5659–67.
Zander T, Scheffler M, Nogova L, Kobe C, Engel-Riedel W, Hellmich M, Papachristou I, Toepelt K, Draube A, Heukamp L, Buettner R, Ko YD, Ullrich RT, Smit E, Boellaard R, Lammertsma AA, Hallek M, Jacobs AH, Schlesinger A, Schulte K, Querings S, Stoelben E, Neumaier B, Thomas RK, Dietlein M, Wolf J. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron emission tomography. J Clin Oncol. 2011;29:1701–8.
Zhao F, Ding G, Huang W, Li M, Fu Z, Yang G, Kong L, Zhang Y, Yu J. FDG-PET Predicts pain response and local control in palliative radiotherapy with or without systemic treatment in patients with bone metastasis from non-small-cell Lung cancer. Clin Lung Cancer. 2015;16:e111–9.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit-sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that none of the authors have any conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Koizumi, T., Fukushima, T., Gomi, D. et al. Correlation of early PET findings with tumor response to molecular targeted agents in patients with advanced driver-mutated non-small cell lung cancer. Med Oncol 34, 169 (2017). https://doi.org/10.1007/s12032-017-1032-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1032-0