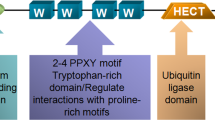
Abstract
C-Jun activation domain-binding protein-1 (Jab1) not only is full but also a subunit (CSN5) of the constitutive photomorphogenesis 9 signalosome (CSN), which is an evolutionarily conserved and multifunctional protein that involves in controlling cellular proliferation and apoptosis, affecting a series of pathways, as well as regulating genomic instability and DNA damage and repair. The CSN is a highly conservative protein from yeast to human and interacts with the cullin-RING family of ubiquitin ligases so that it could be execute a process of removing NEDD8, a ubiquitin-like polypeptide (deneddylase activity). The role of Jab1/CSN5’s multi-function has been proved as being oncogenic in nature, what is more, Jab1/CSN5 has been confirmed by much evidence that it participates in the carcinogenesis progression and is tightly associated with poor prognosis. However, the biologic implication of Jab1/CSN5 activity during the cancer’s development is unclear. We performed a systematic literature review and assessment from PubMed and Medline databases in this article. Jab1/CSN5 is participate in a lot of biologic responses, including cell proliferation, apoptosis, cell cycle regulation, DNA metabolism, invasion, DNA damage and repair, and recurrence. It also promotes cell transformation and tumorigenesis. In this review, we mainly expound the progress in the function and research advances of Jab1/CSN5 and in untangling the Jab1/CSN5 signaling pathway. Based on these bases, its potential as a therapeutic target for cancer can play a greater role in future cancer treatment.


Similar content being viewed by others
References
Chamovitz DA, Wei N, Osterlund MT, von Arnim AG, Staub JM, Matsui M, et al. The COP9 complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell. 1996;86(1):115–21.
Chamovitz DA, Segal D. JAB1/CSN5 and the COP9 signalosome. A complex situation. EMBO Rep. 2001;2(2):96–101. doi:10.1093/embo-reports/kve028.
Gusmaroli G, Figueroa P, Serino G, Deng XW. Role of the MPN subunits in COP9 signalosome assembly and activity, and their regulatory interaction with Arabidopsis Cullin3-based E3 ligases. Plant Cell. 2007;19(2):564–81. doi:10.1105/tpc.106.047571.
Wei N, Deng XW. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–86. doi:10.1146/annurev.cellbio.19.111301.112449.
Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398(6723):160–5. doi:10.1038/18230.
Esteva FJ, Sahin AA, Rassidakis GZ, Yuan LX, Smith TL, Yang Y, et al. Jun activation domain binding protein 1 expression is associated with low p27(Kip1) levels in node-negative breast cancer. Clin Cancer Res. 2003;9(15):5652–9.
Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y, et al. Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res. 2001;7(12):4130–5.
Lee MH, Zhao R, Phan L, Yeung SC. Roles of COP9 signalosome in cancer. Cell Cycle. 2011;10(18):3057–66.
Zhang XC, Chen J, Su CH, Yang HY, Lee MH. Roles for CSN5 in control of p53/MDM2 activities. J Cell Biochem. 2008;103(4):1219–30. doi:10.1002/jcb.21504.
Li J, Gu Z, Li S, Xiao Z, Sun K. Reverse correlation of Jab1 and Smad4 in PANC-1 cells involved in the pathogenesis of pancreatic cancer. Int J Clin Exp Pathol. 2015;8(8):9279–85.
Pan Y, Zhang Q, Atsaves V, Yang H, Claret FX. Suppression of Jab1/CSN5 induces radio- and chemo-sensitivity in nasopharyngeal carcinoma through changes to the DNA damage and repair pathways. Oncogene. 2013;32(22):2756–66. doi:10.1038/onc.2012.294.
Tian L, Peng G, Parant JM, Leventaki V, Drakos E, Zhang Q, et al. Essential roles of Jab1 in cell survival, spontaneous DNA damage and DNA repair. Oncogene. 2010;29(46):6125–37. doi:10.1038/onc.2010.345.
Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383(6599):453–7. doi:10.1038/383453a0.
Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, et al. Jab1 interacts directly with HIF-1α and regulates its stability. J Biol Chem. 2002;277(1):9–12. doi:10.1074/jbc.C100442200.
Adler AS, Lin M, Horlings H, Nuyten DS, van de Vijver MJ, Chang HY. Genetic regulators of large-scale transcriptional signatures in cancer. Nat Genet. 2006;38(4):421–30. doi:10.1038/ng1752.
Guo H, Jing L, Cheng Y, Atsaves V, Lv Y, Wu T, et al. Down-regulation of the cyclin-dependent kinase inhibitor p57 is mediated by Jab1/Csn5 in hepatocarcinogenesis. Hepatology. 2016;63(3):898–913. doi:10.1002/hep.28372.
Xu T, Su B, Wang C, Wang S, Huang H, Pan Y, et al. Molecular markers to assess short-term disease local recurrence in nasopharyngeal carcinoma. Oncol Rep. 2015;33(3):1418–26. doi:10.3892/or.2015.3739.
Zhang SN, Pei DS, Zheng JN. The COP9 signalosome subunit 6 (CSN6): a potential oncogene. Cell Div. 2013;8(1):14. doi:10.1186/1747-1028-8-14.
Wei N, Serino G, Deng XW. The COP9 signalosome: more than a protease. Trends Biochem Sci. 2008;33(12):592–600. doi:10.1016/j.tibs.2008.09.004.
Tran HJ, Allen MD, Lowe J, Bycroft M. Structure of the Jab1/MPN domain and its implications for proteasome function. Biochemistry. 2003;42(39):11460–5. doi:10.1021/bi035033g.
Schwechheimer C, Deng XW. COP9 signalosome revisited: a novel mediator of protein degradation. Trends Cell Biol. 2001;11(10):420–6.
Wolf DA, Zhou C, Wee S. The COP9 signalosome: an assembly and maintenance platform for cullin ubiquitin ligases? Nat Cell Biol. 2003;5(12):1029–33. doi:10.1038/ncb1203-1029.
Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134(6):995–1006. doi:10.1016/j.cell.2008.07.022.
Busch S, Schwier EU, Nahlik K, Bayram O, Helmstaedt K, Draht OW, et al. An eight-subunit COP9 signalosome with an intact JAMM motif is required for fungal fruit body formation. Proc Natl Acad Sci USA. 2007;104(19):8089–94. doi:10.1073/pnas.0702108104.
Rosel D, Kimmel AR. The COP9 signalosome regulates cell proliferation of Dictyostelium discoideum. Eur J Cell Biol. 2006;85(9–10):1023–34. doi:10.1016/j.ejcb.2006.04.006.
Sun J, Liu W, Adams TS, Sun J, Li X, Turner AR, et al. DNA copy number alterations in prostate cancers: a combined analysis of published CGH studies. Prostate. 2007;67(7):692–700. doi:10.1002/pros.20543.
Fejzo MS, Godfrey T, Chen C, Waldman F, Gray JW. Molecular cytogenetic analysis of consistent abnormalities at 8q12-q22 in breast cancer. Genes Chromosomes Cancer. 1998;22(2):105–13.
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32. doi:10.1056/NEJM198809013190901.
Dimova I, Orsetti B, Negre V, Rouge C, Ursule L, Lasorsa L, et al. Genomic markers for ovarian cancer at chromosomes 1, 8 and 17 revealed by array CGH analysis. Tumori. 2009;95(3):357–66.
Shackleford TJ, Claret FX. JAB1/CSN5: a new player in cell cycle control and cancer. Cell Div. 2010;5:26. doi:10.1186/1747-1028-5-26.
Kwok SF, Solano R, Tsuge T, Chamovitz DA, Ecker JR, Matsui M, et al. Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex, and their abundance is differentially affected by the pleiotropic cop/det/fus mutations. Plant Cell. 1998;10(11):1779–90.
Oron E, Mannervik M, Rencus S, Harari-Steinberg O, Neuman-Silberberg S, Segal D, et al. COP9 signalosome subunits 4 and 5 regulate multiple pleiotropic pathways in Drosophila melanogaster. Development. 2002;129(19):4399–409.
Sharon M, Mao H, Erba EB, Stephens E, Zheng N, Robinson CV. Symmetrical modularity of the COP9 signalosome complex suggests its multifunctionality. Structure. 2009;17(1):31–40. doi:10.1016/j.str.2008.10.012.
Cope GA, Deshaies RJ. COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell. 2003;114(6):663–71.
Dubiel W. Resolving the CSN and CAND1 paradoxes. Mol Cell. 2009;35(5):547–9. doi:10.1016/j.molcel.2009.08.011.
Cope GA, Suh GS, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, et al. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science. 2002;298(5593):608–11. doi:10.1126/science.1075901.
Pan Y, Claret FX. Targeting Jab1/CSN5 in nasopharyngeal carcinoma. Cancer Lett. 2012;326(2):155–60. doi:10.1016/j.canlet.2012.07.033.
Adler AS, Littlepage LE, Lin M, Kawahara TL, Wong DJ, Werb Z, et al. CSN5 isopeptidase activity links COP9 signalosome activation to breast cancer progression. Cancer Res. 2008;68(2):506–15. doi:10.1158/0008-5472.CAN-07-3060.
Pan Y, Yang H, Claret FX. Emerging roles of Jab1/CSN5 in DNA damage response, DNA repair, and cancer. Cancer Biol Ther. 2014;15(3):256–62. doi:10.4161/cbt.27823.
Hsu MC, Huang CC, Chang HC, Hu TH, Hung WC. Overexpression of Jab1 in hepatocellular carcinoma and its inhibition by peroxisome proliferator-activated receptor γ ligands in vitro and in vivo. Clin Cancer Res. 2008;14(13):4045–52. doi:10.1158/1078-0432.CCR-07-5040.
Kouvaraki MA, Korapati AL, Rassidakis GZ, Tian L, Zhang Q, Chiao P, et al. Potential role of Jun activation domain-binding protein 1 as a negative regulator of p27kip1 in pancreatic adenocarcinoma. Cancer Res. 2006;66(17):8581–9. doi:10.1158/0008-5472.CAN-06-0975.
Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX. Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1). Cancer Res. 2003;63(11):2977–81.
Osoegawa A, Yoshino I, Kometani T, Yamaguchi M, Kameyama T, Yohena T, et al. Overexpression of Jun activation domain-binding protein 1 in nonsmall cell lung cancer and its significance in p27 expression and clinical features. Cancer. 2006;107(1):154–61. doi:10.1002/cncr.21961.
Yoshida A, Yoneda-Kato N, Kato JY. CSN5 specifically interacts with CDK2 and controls senescence in a cytoplasmic cyclin E-mediated manner. Sci Rep. 2013;3:1054. doi:10.1038/srep01054.
Wang Z, Fukushima H, Inuzuka H, Wan L, Liu P, Gao D, et al. Skp2 is a promising therapeutic target in breast cancer. Front Oncol. 2012;1:57. doi:10.3389/fonc.2011.00057.
Cocklin R, Goebl M. Nutrient sensing kinases PKA and Sch9 phosphorylate the catalytic domain of the ubiquitin-conjugating enzyme Cdc34. PLoS One. 2011;6(11):e27099. doi:10.1371/journal.pone.0027099.
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3(2):231–4.
Guo Y, Sklar GN, Borkowski A, Kyprianou N. Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res. 1997;3(12 Pt 1):2269–74.
Masciullo V, Sgambato A, Pacilio C, Pucci B, Ferrandina G, Palazzo J, et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res. 1999;59(15):3790–4.
Pan Y, Zhang Q, Tian L, Wang X, Fan X, Zhang H, et al. Jab1/CSN5 negatively regulates p27 and plays a role in the pathogenesis of nasopharyngeal carcinoma. Cancer Res. 2012;72(7):1890–900. doi:10.1158/0008-5472.CAN-11-3472.
Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6(12):1229–35. doi:10.1038/ncb1194.
Launay JM, Herve P, Peoc’h K, Tournois C, Callebert J, Nebigil CG, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8(10):1129–35. doi:10.1038/nm764.
Zhang Q, Tian L, Mansouri A, Korapati AL, Johnson TJ, Claret FX. Inducible expression of a degradation-resistant form of p27Kip1 causes growth arrest and apoptosis in breast cancer cells. FEBS Lett. 2005;579(18):3932–40. doi:10.1016/j.febslet.2005.06.012.
Hsu MC, Chang HC, Hung WC. HER-2/neu transcriptionally activates Jab1 expression via the AKT/beta-catenin pathway in breast cancer cells. Endocr Relat Cancer. 2007;14(3):655–67. doi:10.1677/ERC-07-0077.
Fukumoto A, Tomoda K, Yoneda-Kato N, Nakajima Y, Kato JY. Depletion of Jab1 inhibits proliferation of pancreatic cancer cell lines. FEBS Lett. 2006;580(25):5836–44. doi:10.1016/j.febslet.2006.09.042.
Sang MM, Du WQ, Zhang RY, Zheng JN, Pei DS. Suppression of CSN5 promotes the apoptosis of gastric cancer cells through regulating p53-related apoptotic pathways. Bioorg Med Chem Lett. 2015;25(15):2897–901. doi:10.1016/j.bmcl.2015.05.057.
Reed JC. Bcl-2 family proteins. Oncogene. 1998;17(25):3225–36. doi:10.1038/sj.onc.1202591.
Lee YH, Judge AD, Seo D, Kitade M, Gomez-Quiroz LE, Ishikawa T, et al. Molecular targeting of CSN5 in human hepatocellular carcinoma: a mechanism of therapeutic response. Oncogene. 2011;30(40):4175–84. doi:10.1038/onc.2011.126.
Brady HJ, Gil-Gomez G. Bax. The pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol. 1998;30(6):647–50.
Guo B, Godzik A, Reed JC. Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J Biol Chem. 2001;276(4):2780–5. doi:10.1074/jbc.M005889200.
Liu X, Pan Z, Zhang L, Sun Q, Wan J, Tian C, et al. JAB1 accelerates mitochondrial apoptosis by interaction with proapoptotic BclGs. Cell Signal. 2008;20(1):230–40. doi:10.1016/j.cellsig.2007.10.012.
No YR, Lee SJ, Kumar A, Yun CC. HIF1α-induced by Lysophosphatidic acid is stabilized via interaction with MIF and CSN5. PLoS One. 2015;10(9):e0137513. doi:10.1371/journal.pone.0137513.
Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi:10.1038/nri1200.
Burger-Kentischer A, Finkelmeier D, Thiele M, Schmucker J, Geiger G, Tovar GE, et al. Binding of JAB1/CSN5 to MIF is mediated by the MPN domain but is independent of the JAMM motif. FEBS Lett. 2005;579(7):1693–701. doi:10.1016/j.febslet.2005.01.080.
Lechien JR, Kindt N, Costa Pde A, Chantrain G, Preillon J, Laurent G, et al. MIF in head and neck cancer: a new therapeutic target? Rev Laryngol Otol Rhinol. 2013;134(2):67–74.
Wilson JM, Coletta PL, Cuthbert RJ, Scott N, MacLennan K, Hawcroft G, et al. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;129(5):1485–503. doi:10.1053/j.gastro.2005.07.061.
Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, et al. Macrophage migration inhibitory factor promotes tumor invasion and metastasis via the Rho-dependent pathway. Clin Cancer Res. 2005;11(3):1050–8.
Ren Y, Law S, Huang X, Lee PY, Bacher M, Srivastava G, et al. Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann Surg. 2005;242(1):55–63.
del Vecchio MT, Tripodi SA, Arcuri F, Pergola L, Hako L, Vatti R, et al. Macrophage migration inhibitory factor in prostatic adenocarcinoma: correlation with tumor grading and combination endocrine treatment-related changes. Prostate. 2000;45(1):51–7.
Han I, Lee MR, Nam KW, Oh JH, Moon KC, Kim HS. Expression of macrophage migration inhibitory factor relates to survival in high-grade osteosarcoma. Clin Orthop Relat Res. 2008;466(9):2107–13. doi:10.1007/s11999-008-0333-1.
Meyer-Siegler KL, Vera PL, Iczkowski KA, Bifulco C, Lee A, Gregersen PK, et al. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun. 2007;8(8):646–52. doi:10.1038/sj.gene.6364427.
Savaskan NE, Fingerle-Rowson G, Buchfelder M, Eyupoglu IY. Brain miffed by macrophage migration inhibitory factor. Int J Cell Biol. 2012;2012:139573. doi:10.1155/2012/139573.
Turtzo LC, Li J, Persky R, Benashski S, Weston G, Bucala R, et al. Deletion of macrophage migration inhibitory factor worsens stroke outcome in female mice. Neurobiol Dis. 2013;54:421–31. doi:10.1016/j.nbd.2013.01.016.
Rendon BE, Willer SS, Zundel W, Mitchell RA. Mechanisms of macrophage migration inhibitory factor (MIF)-dependent tumor microenvironmental adaptation. Exp Mol Pathol. 2009;86(3):180–5. doi:10.1016/j.yexmp.2009.01.001.
Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408(6809):211–6. doi:10.1038/35041591.
Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26(35):5046–59. doi:10.1038/sj.onc.1210318.
Winner M, Koong AC, Rendon BE, Zundel W, Mitchell RA. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res. 2007;67(1):186–93. doi:10.1158/0008-5472.CAN-06-3292.
Kleemann R, Grell M, Mischke R, Zimmermann G, Bernhagen J. Receptor binding and cellular uptake studies of macrophage migration inhibitory factor (MIF): use of biologically active labeled MIF derivatives. J Interferon Cytokine Res. 2002;22(3):351–63. doi:10.1089/107999002753675785.
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342(6250):705–8. doi:10.1038/342705a0.
Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1(14):993–1000.
Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275(12):8945–51.
Asano K, Vornlocher HP, Richter-Cook NJ, Merrick WC, Hinnebusch AG, Hershey JW. Structure of cDNAs encoding human eukaryotic initiation factor 3 subunits. Possible roles in RNA binding and macromolecular assembly. J Biol Chem. 1997;272(43):27042–52.
Bech-Otschir D, Kraft R, Huang X, Henklein P, Kapelari B, Pollmann C, et al. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20(7):1630–9. doi:10.1093/emboj/20.7.1630.
Larsen M, Hog A, Lund EL, Kristjansen PE. Interactions between HIF-1 and Jab1: balancing apoptosis and adaptation. Outline of a working hypothesis. Adv Exp Med Biol. 2005;566:203–11. doi:10.1007/0-387-26206-7_28.
Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13(1):29–37. doi:10.1006/scdb.2001.0287.
Kondo K, Kim WY, Lechpammer M, Kaelin WG Jr. Inhibition of HIF2α is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1(3):E83. doi:10.1371/journal.pbio.0000083.
Bemis L, Chan DA, Finkielstein CV, Qi L, Sutphin PD, Chen X, et al. Distinct aerobic and hypoxic mechanisms of HIF-α regulation by CSN5. Genes Dev. 2004;18(7):739–44. doi:10.1101/gad.1180104.
Huang J, Yuan H, Lu C, Liu X, Cao X, Wan M. Jab1 mediates protein degradation of the Rad9-Rad1-Hus1 checkpoint complex. J Mol Biol. 2007;371(2):514–27. doi:10.1016/j.jmb.2007.05.095.
Doronkin S, Djagaeva I, Beckendorf SK. CSN5/Jab1 mutations affect axis formation in the Drosophila oocyte by activating a meiotic checkpoint. Development. 2002;129(21):5053–64.
Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, et al. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell. 2003;113(3):357–67.
Higa LA, Mihaylov IS, Banks DP, Zheng J, Zhang H. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat Cell Biol. 2003;5(11):1008–15. doi:10.1038/ncb1061.
Parrilla-Castellar ER, Arlander SJ, Karnitz L. Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair. 2004;3(8–9):1009–14. doi:10.1016/j.dnarep.2004.03.032.
Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69(3):457–70.
Tsuzuki T, Fujii Y, Sakumi K, Tominaga Y, Nakao K, Sekiguchi M, et al. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc Natl Acad Sci USA. 1996;93(13):6236–40.
Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shinohara A, Ogawa H, et al. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998;17(2):598–608. doi:10.1093/emboj/17.2.598.
Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. Trends Cell Biol. 2012;22(4):211–9. doi:10.1016/j.tcb.2011.11.006.
Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18(34):4808–18. doi:10.1038/sj.onc.1203078.
Doronkin S, Djagaeva I, Beckendorf SK. The COP9 signalosome promotes degradation of Cyclin E during early Drosophila oogenesis. Dev Cell. 2003;4(5):699–710.
Campaner S, Doni M, Hydbring P, Verrecchia A, Bianchi L, Sardella D, et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat Cell Biol. 2010;12(1):54–9. doi:10.1038/ncb2004 (Sup pp. 1–14).
Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–33. doi:10.1016/j.cell.2007.07.003.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81572349) and the Science and Technology Department of Jiangsu Province (BK20130231, BK20141149).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wang, L., Zheng, JN. & Pei, DS. The emerging roles of Jab1/CSN5 in cancer. Med Oncol 33, 90 (2016). https://doi.org/10.1007/s12032-016-0805-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0805-1