Abstract
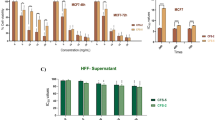
Texosomes, nano-endosomal vesicles, are candidates for cancer immunotherapy due to their immunostimulating properties. We designed a new structure based on texosome and staphylococcal enterotoxin B (SEB) and assessed its cytotoxic impact on an ovarian cell line. Texosomes were isolated from tumor cells, and SEB was anchored onto by protein transfer method. MTT assay and Hoechst staining were used to identify the cytotoxic and apoptotic effects of this compound on treated cells with different concentrations of texosome–SEB (TEX–SEB). Moreover, the expression rate of bcl-2, bax, bak, bcl-xl and the activity of caspase-3 and caspase-9 were investigated. Treatments of the cells with 0.5, 2.5 and 10 μg/100 μl TEX–SEB were significantly cytotoxic within 24 h (p < 0.001). Hoechst staining revealed that all tested concentrations caused apoptosis after 24 h compared with the control cells (p < 0.001). Furthermore, it was found that treatment with all examined concentrations of TEX–SEB enhanced caspase-9 activity after 24 and 48 h, while caspase-3 activity was increased upon treatment with only 0.5 and 2.5 μg/100 μl of TEX–SEB after 24 h (p < 0.001). None of the concentrations of TEX–SEB affected the expression of the cancer-promoting genes. Our construct, the TEX–SEB, is a new model being able to create cytostatic properties on cancer cells.








Similar content being viewed by others
References
Peng P, Yan Y, Keng S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep. 2011;25(3):749–62. doi:10.3892/or.2010.1119.
Alberts DS. Treatment of refractory and recurrent ovarian cancer. Semin Oncol. 1999;26(1 Suppl 1):8–14.
McGuire WP, Ozols RF. Chemotherapy of advanced ovarian cancer. Semin Oncol. 1998;25(3):340–8.
Rieger J, Freichels H, Imberty A, Putaux JL, Delair T, Jérôme C, et al. Polyester nanoparticles presenting mannose residues: toward the development of new vaccine delivery systems combining biodegradability and targeting properties. Biomacromolecules. 2009;10(3):651–7. doi:10.1021/bm801492c.
Hosseini HM, Fooladi AA, Nourani MR, Ghanezadeh F. Role of Exosome in infectious disease. Inflamm Allergy Drug Targets. 2013;12(1):29–37.
Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–81. doi:10.1111/j.1600-0854.2008.00734.x.
Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114(4):613–22. doi:10.1002/ijc.20757.
Tan A, De La Peña H, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. Int J Nanomed. 2010;5:889–900. doi:10.2147/ijn.s13402.
Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125(5):1016–26. doi:10.1002/ijc.24375.
Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V, et al. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22(9):3358–69. doi:10.1096/fj.07-102855.
Mahmoodzadeh Hosseini H, Ali Imani Fooladi A, Soleimanirad J, Reza Nourani M, Mahdavi M. Exosome/staphylococcal enterotoxin B, an anti tumor compound against pancreatic cancer. J BUON. 2014;19(2):440–8.
Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J, Nourani MR, Davaran S, Mahdavi M. Staphylococcal entorotoxin B anchored exosome induces apoptosis in negative estrogen receptor breast cancer cells. Tumour Biol. 2014;35(4):3699–707. doi:10.1007/s13277-013-1489-1.
Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci. 1989;86(22):8941–5.
Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, et al. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244(4906):811–3.
Imani Fooladi AA, Sattari M, Hassan ZM, Mahdavi M, Azizi T, Horii A. In vivo induction of necrosis in mice fibrosarcoma via intravenous injection of type B staphylococcal enterotoxin. Biotechnol Lett. 2008;30(12):2053–9. doi:10.1007/s10529-008-9805-3.
Imani Fooladi AA, Sattari M, Nourani MR. Synergistic effects between Staphylococcal enterotoxin type B and monophosphoryl lipid A against mouse fibrosarcoma. J BUON. 2010;15(2):340–7.
Fooladi AAI, Sattari M, Nourani MR. Study of T-cell stimulation and cytokine release induced by Staphylococcal enterotoxin type B and monophosphoryl lipid A. Arch Med Sci. 2009;3:335–41.
Higgs BW, Dileo J, Chang WE, Smith HB, Peters OJ, Hammamieh R, et al. Modeling the effects of a Staphylococcal Enterotoxin B (SEB) on the apoptosis pathway. BMC Microbiol. 2006;6:48. doi:10.1186/1471-2180-6-48.
Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–48. doi:10.1007/s00262-011-0979-5.
McHugh RS, Nagarajan S, Wang YC, Sell KW, Selvaraj P. Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer Res. 1999;59(10):2433–7.
Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, et al. Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis. 2005;35(2):158–68. doi:10.1016/j.bcmd.2005.07.003.
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–7.
Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Möbius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–72. doi:10.1074/jbc.M207550200.
Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med. 2007;85(5):511–21. doi:10.1007/s00109-006-0154-1.
Nagarajan S, Anderson M, Ahmed SN, Sell KW, Selvaraj P. Purification and optimization of functional reconstitution on the surface of leukemic cell lines of GPI-anchored Fc gamma receptor III. J Immunol Methods. 1995;184(2):241–51.
Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258(6):479–517. doi:10.1111/j.1365-2796.2005.01570.x.
Sharief M, Gani ZH. Garden cress lepidium sativum seeds as oral contraceptive plant in mice. Saudi Med J. 2004;25(7):965–6.
Ferrari D, Pinton P, Campanella M, Callegari MG, Pizzirani C, Rimessi A, et al. Functional and structural alterations in the endoplasmic reticulum and mitochondria during apoptosis triggered by C2-ceramide and CD95/APO-1/FAS receptor stimulation. Biochem Biophys Res Commun. 2010;391(1):575–81. doi:10.1016/j.bbrc.2009.11.101.
Wlodkowic D, Skommer J, McGuinness D, Hillier C, Darzynkiewicz Z. ER-Golgi network—a future target for anti-cancer therapy. Leuk Res. 2009;33(11):1440–7. doi:10.1016/j.leukres.2009.05.025.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmoodzadeh Hosseini, H., Soleimanirad, J., Mehdizadeh Aghdam, E. et al. Texosome-anchored superantigen triggers apoptosis in original ovarian cancer cells. Med Oncol 32, 409 (2015). https://doi.org/10.1007/s12032-014-0409-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0409-6