Abstract
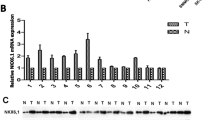
Karyopherin α2 (KPNA2) functions as an adaptor that transports several proteins to the nucleus. Emerging evidence suggests that KPNA2 plays a crucial role in oncogenesis and early recurrence. In the present study, we evaluated the expression pattern of KPNA2 in 221 hepatocellular carcinoma (HCC) specimens and matching adjacent, non-tumorous tissues (NT) by immunohistochemical assays. We found that nuclear KPNA2 expression was significantly upregulated (30.3 %, 67/221) in HCC tissues; however, no nuclear expression of KPNA2 in NT tissues was observed. A correlation analysis demonstrated that nuclear KPNA2 expression was positively associated with serum AFP level, tumor differentiation, vascular invasion, BCLC stage and early recurrence (all p < 0.05). Nuclear KPNA2 expression was associated with a poor prognosis in HCC patients. Univariate and multivariate analyses demonstrated that KPNA2 was an independent prognostic factor for both overall survival (p < 0.001) and time to recurrence (p < 0.001) in HCC patients. Furthermore, in a validation cohort, nuclear expression of KPNA2 was observed in 16 of 47 (34.0 %) small hepatocellular carcinoma patients. Importantly, the risk of recurrence associated with nuclear KPNA2 expression (9/16, 56.2 %) was significantly higher than the risk associated with an absence of nuclear KPNA2 expression (6/31, 19.3 %; p = 0.01). Our results demonstrate that nuclear KPNA2 expression is a poor prognostic biomarker for HCC, especially for early-stage HCC.


Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107.
Hong J, Hu K, Yuan Y, Sang Y, Bu Q, Chen G, et al. CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. J Clin Investig. 2012;122(6):2165–75. doi:10.1172/jci61380.
Suh SW, Lee KW, Lee JM, You T, Choi Y, Kim H, et al. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J Hepatol. 2014;. doi:10.1016/j.jhep.2014.01.027.
Kelley JB, Talley AM, Spencer A, Gioeli D, Paschal BM. Karyopherin alpha7 (KPNA7), a divergent member of the importin alpha family of nuclear import receptors. BMC Cell Biol. 2010;11:63. doi:10.1186/1471-2121-11-63.
Radu A, Blobel G, Moore MS. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995;92(5):1769–73.
Weis K, Mattaj IW, Lamond AI. Identification of hSRP1 alpha as a functional receptor for nuclear localization sequences. Science (New York, NY). 1995;268(5213):1049–53.
Weis K, Ryder U, Lamond AI. The conserved amino-terminal domain of hSRP1 alpha is essential for nuclear protein import. EMBO J. 1996;15(8):1818–25.
Hogarth CA, Calanni S, Jans DA, Loveland KL. Importin alpha mRNAs have distinct expression profiles during spermatogenesis. Dev Dyn. 2006;235(1):253–62. doi:10.1002/dvdy.20569.
Hall MN, Griffin CA, Simionescu A, Corbett AH, Pavlath GK. Distinct roles for classical nuclear import receptors in the growth of multinucleated muscle cells. Dev Biol. 2011;357(1):248–58. doi:10.1016/j.ydbio.2011.06.032.
Cabot RA, Prather RS. Cleavage stage porcine embryos may have differing developmental requirements for karyopherins alpha2 and alpha3. Mol Reprod Dev. 2003;64(3):292–301. doi:10.1002/mrd.10238.
Fischer N, Kremmer E, Lautscham G, Mueller-Lantzsch N, Grasser FA. Epstein-Barr virus nuclear antigen 1 forms a complex with the nuclear transporter karyopherin alpha2. J Biol Chem. 1997;272(7):3999–4005.
Qu Q, Sawa H, Suzuki T, Semba S, Henmi C, Okada Y, et al. Nuclear entry mechanism of the human polyomavirus JC virus-like particle: role of importins and the nuclear pore complex. J Biol Chem. 2004;279(26):27735–42. doi:10.1074/jbc.M310827200.
Merle E, Rose RC, LeRoux L, Moroianu J. Nuclear import of HPV11 L1 capsid protein is mediated by karyopherin alpha2beta1 heterodimers. J Cell Biochem. 1999;74(4):628–37.
Noetzel E, Rose M, Bornemann J, Gajewski M, Knuchel R, Dahl E. Nuclear transport receptor karyopherin-alpha2 promotes malignant breast cancer phenotypes in vitro. Oncogene. 2012;31(16):2101–14. doi:10.1038/onc.2011.403.
Altan B, Yokobori T, Mochiki E, Ohno T, Ogata K, Ogawa A, et al. Nuclear karyopherin-alpha2 expression in primary lesions and metastatic lymph nodes was associated with poor prognosis and progression in gastric cancer. Carcinogenesis. 2013;34(10):2314–21. doi:10.1093/carcin/bgt214.
Wang CI, Chien KY, Wang CL, Liu HP, Cheng CC, Chang YS, et al. Quantitative proteomics reveals regulation of karyopherin subunit alpha-2 (KPNA2) and its potential novel cargo proteins in nonsmall cell lung cancer. Mol Cell Proteomics. 2012;11(11):1105–22. doi:10.1074/mcp.M111.016592.
Zheng M, Tang L, Huang L, Ding H, Liao WT, Zeng MS, et al. Overexpression of karyopherin-2 in epithelial ovarian cancer and correlation with poor prognosis. Obstet Gynecol. 2010;116(4):884–91. doi:10.1097/AOG.0b013e3181f104ce.
Jensen JB, Munksgaard PP, Sorensen CM, Fristrup N, Birkenkamp-Demtroder K, Ulhoi BP, et al. High expression of karyopherin-alpha2 defines poor prognosis in non-muscle-invasive bladder cancer and in patients with invasive bladder cancer undergoing radical cystectomy. Eur Urol. 2011;59(5):841–8. doi:10.1016/j.eururo.2011.01.048.
He L, Zhou X, Qu C, Tang Y, Zhang Q, Hong J. Serglycin (SRGN) overexpression predicts poor prognosis in hepatocellular carcinoma patients. Med Oncol (Northwood, London, England). 2013;30(4):707. doi:10.1007/s12032-013-0707-4.
He L, Zhou X, Qu C, Hu L, Tang Y, Zhang Q, et al. Musashi2 predicts poor prognosis and invasion in hepatocellular carcinoma by driving epithelial-mesenchymal transition. J Cell Mol Med. 2014;18(1):49–58. doi:10.1111/jcmm.12158.
Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359(19):2045–7. doi:10.1056/NEJMe0807581.
Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16(4):792–4. doi:10.1245/s10434-009-0330-y.
Hong J, Yuan Y, Wang J, Liao Y, Zou R, Zhu C, et al. Expression of variant isoforms of the tyrosine kinase SYK determines the prognosis of hepatocellular carcinoma. Cancer Res. 2014;74(6):1845–56. doi:10.1158/0008-5472.can-13-2104.
Radu A, Moore MS, Blobel G. The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell. 1995;81(2):215–22.
Dankof A, Fritzsche FR, Dahl E, Pahl S, Wild P, Dietel M, et al. KPNA2 protein expression in invasive breast carcinoma and matched peritumoral ductal carcinoma in situ. Virchows Arch. 2007;451(5):877–81. doi:10.1007/s00428-007-0513-5.
Acknowledgments
We acknowledge National Natural Science Foundation of China (81201634); Basilic Special Financial of Affiliated Tumor Hospital Guangzhou Medical University (2011-yz-03); Ph.D. Startup Foundation of Guangzhou Medical University (2012C65).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, P., Tang, Y., He, L. et al. Aberrant expression of nuclear KPNA2 is correlated with early recurrence and poor prognosis in patients with small hepatocellular carcinoma after hepatectomy. Med Oncol 31, 131 (2014). https://doi.org/10.1007/s12032-014-0131-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0131-4