Abstract
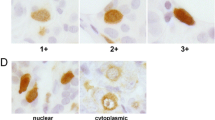
The aim of this study was to evaluate protein expression of Karyopherin alpha 2 (KPNA2) in invasive breast cancer and matched ductal carcinoma in situ (DCIS) and to correlate it with clinicopathological data, including patient survival. KPNA2 protein expression was assessed by immunohistochemistry in breast tissue samples, containing invasive carcinomas, DCIS, and adjacent histologically benign breast tissues. A polyclonal goat KPNA2 antibody was used for immunostaining of 83 clinicopathologically characterized cases. For statistical analysis, staining of at least 10% of nuclei was considered KPNA2 positive. Immunohistochemical detection of KPNA2 in invasive carcinoma showed a significant correlation with higher tumor stage, positive lymph node status, higher tumor grade, and negative ER status. Concordantly, KPNA2-positive tumors (31.3%) showed significantly shorter disease-free survival times (69 months vs 118 months; p = 0.007). KPNA2 protein expression was also detected in DCIS (21.3%) adjacent to invasive tumor and correlated with nuclear grade (p = 0.013). Expression of KPNA2 in invasive breast cancer correlates with conventional prognostic parameters and shorter disease-free survival. Additionally, KPNA2 is overexpressed in DCIS, particularly high grade lesions, which emphasizes its potential role in carcinogenesis of invasive breast carcinomas.


Similar content being viewed by others
References
Chook YM, Blobel G (2001) Karyopherins and nuclear import. Curr Opin Struct Biol 11(6):703–715
Dahl E, Kristiansen G, Gottlob K, Klaman I, Ebner E, Hinzmann B, Hermann K, Pilarsky C, Durst M, Klinkhammer-Schalke M, Blaszyk H, Knuechel R, Hartmann A, Rosenthal A, Wild PJ (2006) Molecular profiling of laser-microdissected matched tumor and normal breast tissue identifies karyopherin alpha2 as a potential novel prognostic marker in breast cancer. Clin Cancer Res 12(13):3950–3960
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57(1):43–66
Kim IS, Kim DH, Han SM, Chin MU, Nam HJ, Cho HP, Choi SY, Song BJ, Kim ER, Bae YS, Moon YH (2000) Truncated form of importin alpha identified in breast cancer cell inhibits nuclear import of p53. J Biol Chem 275(30):23139–23145
Klein A, Wessel R, Graessmann M, Jürgens M, Petersen I, Schmutzler R, Niederacher D, Arnold N, Meindl A, Scherneck S, Seitz S, Graessmann A (2007) Comparison of gene expression data from human and mouse breast cancers: identification of a conserved breast tumor gene set. Int J Cancer 121(3):683–688
Klopocki E, Kristiansen G, Wild PJ, Klaman I, Castanos-Velez E, Singer G, Stohr R, Simon R, Sauter G, Leibiger H, Essers L, Weber B, Hermann K, Rosenthal A, Hartmann A, Dahl E (2004) Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol 25(3):641–649
Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schluns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H, Dietel M (2003) CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res 9(13):4906–4913
Manders P, Tjan-Heijnen VC, Span PN, Grebenchtchikov N, Geurts-Moespot A, van Tienoven DT, Beex LV, Sweep FC (2004) Complex of urokinase-type plasminogen activator with its type 1 inhibitor predicts poor outcome in 576 patients with lymph node-negative breast carcinoma. Cancer 101(3):486–494
Nakopoulou L, Giannopoulou I, Stefanaki K, Panayotopoulou E, Tsirmpa I, Alexandrou P, Mavrommatis J, Katsarou S, Davaris P (2002) Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol 197(3):307–313
Poon IK, Jans DA (2005) Regulation of nuclear transport: central role in development and transformation? Traffic 6(3):173–186
Rudland PS, Platt-Higgins A, El-Tanani M, De Silva Rudland S, Barraclough R, Winstanley JH, Howitt R, West CR (2002) Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res 62(12):3417–3427
Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ, Duffy MJ (2006) Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol 17(4):597–604
Sobin LH, Fleming ID (1997) TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80:1803–1804
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Span PN, Tjan-Heijnen VC, Manders P, van Tienoven D, Lehr J, Sweep FC (2006) High survivin predicts a poor response to endocrine therapy, but a good response to chemotherapy in advanced breast cancer. Breast Cancer Res Treat 98(2):223–230
Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, Maurer R, Metzger U, von Castelberg B, Bart R, Stopatschinskaya S, Kochli OR, Haas P, Mross F, Zuber M, Dietrich H, Bischoff S, Mirlacher M, Sauter G, Gastl G (2004) High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat 86(3):207–213
Thakur S, Zhang HB, Peng Y, Le H, Carroll B, Ward T, Yao J, Farid LM, Couch FJ, Wilson RB, Weber BL (1997) Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol 17(1):444–452
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365(9460):671–679
Yousef GM, Scorilas A, Kyriakopoulou LG, Rendl L, Diamandis M, Ponzone R, Biglia N, Giai M, Roagna R, Sismondi P, Diamandis EP (2002) Human kallikrein gene 5 (KLK5) expression by quantitative PCR: an independent indicator of poor prognosis in breast cancer. Clin Chem 48(8):1241–1250
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dankof, A., Fritzsche, F.R., Dahl, E. et al. KPNA2 protein expression in invasive breast carcinoma and matched peritumoral ductal carcinoma in situ. Virchows Arch 451, 877–881 (2007). https://doi.org/10.1007/s00428-007-0513-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0513-5