Abstract
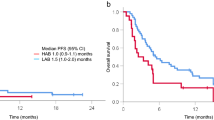
This two-arm, randomised, multicentre, open-label, phase IIIb study investigated the safety and efficacy of a 3-h catumaxomab infusion with/without prednisolone premedication to reduce catumaxomab-related adverse events. Patients with malignant ascites due to epithelial cancer received four 3-h intraperitoneal catumaxomab infusions with/without intravenous prednisolone (25 mg) premedication before each infusion. The primary safety endpoint was a composite safety score calculated from the incidence and intensity of the most frequent catumaxomab-related adverse events (pyrexia, nausea, vomiting and abdominal pain). Puncture-free survival (PuFS) was a co-primary endpoint. Time to next puncture (TTPu) and overall survival (OS) were secondary endpoints. Prednisolone premedication did not result in a significant reduction in the main catumaxomab-related adverse events. The mean composite safety score was comparable in both arms (catumaxomab plus prednisolone, 4.1; catumaxomab, 3.8; p = 0.383). Median PuFS (30 vs. 37 days) and TTPu (78 vs. 102 days) were shorter in the catumaxomab plus prednisolone arm than in the catumaxomab arm, but the differences were not statistically significant (p = 0.402 and 0.599, respectively). Median OS was longer in the catumaxomab plus prednisolone arm than in the catumaxomab arm (124 vs. 86 days), but the difference was not statistically significant (p = 0.186). The superiority of catumaxomab plus prednisolone versus catumaxomab alone could not be proven for the primary endpoint. Prednisolone did not result in a significant reduction in the main catumaxomab-related adverse events. The study confirms the safety and efficacy of catumaxomab administered as four 3-h intraperitoneal infusions for the treatment of malignant ascites.


Similar content being viewed by others
Abbreviations
- CASIMAS:
-
Catumaxomab safety with intraperitoneal infusion in malignant ascites
- EpCAM:
-
Epithelial cell-adhesion molecule
- OS:
-
Overall survival
- PuFS:
-
Puncture-free survival
- QoL:
-
Quality of life
- TTPu:
-
Time to next puncture
- ULN:
-
Upper limit of normal
References
Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg. 2004;198:999–1011.
Ayantunde AA, Parsons S. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–9.
Tamsma J. The pathogenesis of malignant ascites. Cancer Treat Res. 2007;134:109–18.
Mackey JR, Venner PM. Malignant ascites: demographics, therapeutic efficacy and predictors of survival. Can J Oncol. 1996;6:474–80.
Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: a multi-institutional retrospective analysis in 52 patients. Surg Oncol. 2009;100:331–4.
Parsons SL, Watson SA, Steele RJC. Malignant ascites. Br J Surg. 1996;83:6–14.
Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer. 2006;42:589–97.
Cavazzoni E, Bugiantella W, Graziosi L, Franceschini MS, Donini A. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol. 2013;18:1–9.
Barni S, Cabiddu M, Ghilardi M, Petrelli F. A novel perspective for an orphan problem: old and new drugs for the medical management of malignant ascites. Crit Rev Oncol Hematol. 2011;79:144–53.
Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res. 2009;29:3353–9.
Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–67.
Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–34.
Ruf P, Gires O, Jäger M, Fellinger K, Atz J, Lindhofer H. Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer. Br J Cancer. 2007;97:315–21.
Zeidler R, Mysliwietz J, Csánady M, Walz A, Ziegler I, Schmitt B, Wollenberg B, Lindhofer H. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261–6.
Jäger M, Schobert A, Ruf P, Hess J, Hennig M, Schmalfeldt B, Wimberger P, Ströhlein M, Theissen B, Heiss MM, Lindhofer H. Immunomonitoring results of a phase II/III study of malignant ascites patients treated with the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3). Cancer Res. 2012;72:24–32.
Riesenberg R, Buchner A, Pohla H, Lindhofer H. Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (αEp-CAM × αCD3). J Histochem Cytochem. 2001;49:911–7.
Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H. Simultaneous activation of T-cells and accessory cells by a new class of intact bispecific antibody results in efficient tumour cell killing. J Immunol. 1999;163:1246–52.
Went P, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCAM protein expression in human carcinomas. Hum Pathol. 2004;35:122–8.
Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G, Baeuerle PA. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128–35.
Spizzo G, Fong D, Wurm M, Ensinger C, Obrist P, Hofer C, Mazzoleni G, Gastl G, Went P. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. Clin Pathol. 2011;64:415–20.
Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75.
Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–71.
Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5.
Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, Maurer R, Metzger U, von Castelberg B, Bart R, Stopatschinskaya S, Köchli OR, Haas P, Mross F, Zuber M, Dietrich H, Bischoff S, Mirlacher M, Sauter G, Gastl G. High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat. 2004;86:207–13.
Davidson B, Risberg B, Kristensen G, Kvalheim G, Emilsen E, Bjåmer A, Berner A. Detection of cancer cells in effusions from patients diagnosed with gynaecological malignancies: evaluation of five epithelial markers. Virchows Arch. 1999;435:43–9.
Passebosc-Faure K, Li G, Lambert C, Cottier M, Gentil-Perret A, Fournel P, Pérol M, Genin C. Evaluation of a panel of molecular markers for the diagnosis of malignant serous effusions. Clin Cancer Res. 2005;11:6862–7.
Burges A, Wimberger P, Kümper C, Gorbounova V, Sommer H, Schmalfeldt B, Pfisterer J, Lichinitser M, Makhson A, Moiseyenko V, Lahr A, Schulze E, Jäger M, Ströhlein MA, Heiss MM, Gottwald T, Lindhofer H, Kimmig R. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13:3899–905.
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–21.
Wimberger P, Gilet H, Gonschior AK, Heiss MM, Moehler M, Oskay-Oezcelik G, Al-Batran SE, Schmalfeldt B, Schmittel A, Schulze E, Parsons SL. Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol. 2012;23:1979–85.
Walz A, Andratschke M, Wollenberg B, Lindhofer H, Zeidler R. Prednisolone reduces TNF-alpha release by PBMCs activated with a trifunctional bispecific antibody but not their anti-tumor activity. Anticancer Res. 2005;25:4239–43.
Berek JS, Edwards RP, Parker L, DeMars LR, Herzog TJ, Lentz SS, Morris R, Akerley WL, Holloway RW, Method M, Plaxe SC, Walker JL, Schindler T, Schulze E, Krasner CN. Catumaxomab treatment of malignant ascites in patients with chemotherapy-refractory ovarian cancer: a phase II study. J Clin Oncol. 2011;29(Suppl.) (abstract 5048).
Sehouli J, Reinthaller A, Marth C, Reimer D, Reimer T, Stummvoll W, Angleitner-Boubenizek L, Lehnert B, Marquardt M, Essing MM, Chekerov R. Intra- and post-operative catumaxomab in patients with epithelial ovarian cancer: two-year efficacy results from a multicenter, single-arm, phase II study. J Clin Oncol. 2011;29(Suppl.) (Abstract 5082).
Baumann K, Pfisterer J, Wimberger P, Burchardi N, Kurzeder C, du Bois A, Loibl S, Sehouli J, Huober J, Schmalfeldt B, Vergote I, Lück HJ, Wagner U. Intraperitoneal treatment with the trifunctional bispecific antibody catumaxomab in patients with platinum-resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecol Oncol. 2011;123:27–32.
Acknowledgments
The authors would like to thank all the investigators who participated in the study: Dr. J. M. Aranda Lara, Cordoba, Spain; Prof. S. Barni, Treviglio, Italy; Dr. K. Baumann, Marburg, Germany; Dr. A. Block, Hamburg, Germany; Dr. V. Bondar, Donetsk, Ukraine; Prof. I. Bondarenko, Dnepropetrovsk, Ukraine; Dr. M. Candiani, Milan, Italy; Dr. A. Casado, Madrid, Spain; Dr. P. Chollet, Clermont-Ferrand, France; Prof. G. Colucci, Bari, Italy; Prof. P. Conte, Modena, Italy; Dr. T. Delaunoit, Haine St Paul, Belgium; Dr. J. M. del Campo, Barcelona, Spain; Dr. G. Demolin, Liege, Belgium; Dr. X. Durando, Clermont-Ferrand, France; Prof. A. Ferrari, Milan, Italy; Dr. E. François, Nice, France; Dr. A. Gambino, Brescia, Italy; Dr. A. Goncalves, Marseille, France; Dr. A. González del Alba, Palma, Majorca; Dr. A. González Martín, Madrid, Spain; Dr. J.-P. Guastalla, Lyon, France; Dr. E. Guerra Alía, Madrid, Spain; Dr. H. Havsteen, Herlev, Denmark; Prof. V. Heinemann, Munich, Germany; Dr. C. Kahl, Magdeburg, Germany; Dr. E. Kettner, Magdeburg, Germany; Dr. P. Klare, Berlin, Germany; Dr. S. Kubicka, Hannover, Germany; Prof. R. Labianca, Bergamo, Italy; Dr. R. Lopéz, Santiago de Compostela, Spain; Dr. S. Lorenzen, Heidelberg, Germany; Dr. D. Lüftner, Berlin, Germany; Dr. E. Luporsi, Nancy, France; Prof. A. Martoni, Bologna, Italy; Dr. B. Massuti, Alicante, Spain; Dr. K. Meunier, Angers, France; Prof. S. Pecorelli, Brescia, Italy; Dr. S. Pignata, Naples, Italy; Prof. P. Piso, Regensburg, Germany; Dr. E. Polycarpe, Angers, France; Dr. F. Popp, Regensburg, Germany; Dr. A. Poveda, Valencia, Spain; Dr. E. Rota Caremoli, Bergamo, Italy; Prof. G. Salerno, Pisa, Italy; Prof. B. Schmalfeldt, Munich, Germany; Dr. G. Schuch, Hamburg, Germany; Dr. E. Sevin, Caen, France; Prof. S. Siena, Milan, Italy; Dr. D. Spaeth, Nancy, France; Prof. M. Stahl, Essen, Germany; Dr. W. Stummvoll, Linz, Austria; Dr. H. Trum, Amsterdam, The Netherlands; Dr. W. M. Van Baal, Amsterdam, The Netherlands; Dr. F. Viret, Marseille, France and Dr. A. Vogel, Hannover, Germany. The authors would also like to thank the freelance medical writer Kevin De-Voy (funded by Fresenius Biotech GmbH) for his writing support.
Conflict of interest
Klaus Pietzner received honoraria for scientific lectures. Hilke Friccius-Quecke is employee of Neovii Biotech (former Fresenius Biotech). All other authors do not report a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jalid Sehouli and Klaus Pietzner have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Sehouli, J., Pietzner, K., Wimberger, P. et al. Catumaxomab with and without prednisolone premedication for the treatment of malignant ascites due to epithelial cancer: results of the randomised phase IIIb CASIMAS study. Med Oncol 31, 76 (2014). https://doi.org/10.1007/s12032-014-0076-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0076-7