Abstract
Background
Cell-free and concentrated ascites reinfusion therapy (CART) has been suggested to be able to treat malignant ascites more safely and effectively with chemotherapy because of its ability to retain serum protein and albumin. Although the characteristics of cancer types and CART and the clinical implications of combination therapy with antitumor agents are becoming widespread, there are limited reports on its efficacy and complications.
Methods
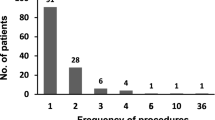
In this prospective observational national post-marketing study, 128 patients with malignancies received 300 CART sessions at 22 centers. After excluding other malignancies, the patients were divided into four groups: gynecological malignancies with chemotherapy (GYC+; 18 cases and 36 times) and without chemotherapy (GYC−; 35 cases and 52 times), and gastrointestinal malignancies with chemotherapy (GIC+; 8 cases and 16 times) and without chemotherapy (20 cases and 58 times).
Results
There were significant reductions in the body weight in all groups and significant reductions in abdominal circumference and significant improvements in the diet and Eastern Cooperative Oncology Group performance status only in the GYC+ group. The total serum protein and albumin increased significantly in all groups, except for the GIC+ group, before and after CART. There was no significant difference in the presence or absence of antitumor medication.
Conclusion
With CART, there were differences in the improvement of the clinical symptoms between malignancy groups. The combination of CART and antineoplastic agents may be as safe as CART alone in cases of exudative malignant ascites.


Similar content being viewed by others
References
Zaak D, Paquet KJ, Kuhn R (2001) Prospective study comparing human albumin vs. reinfusion of ultrafiltrate-ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Z Gastroenterol 39:5–10. https://doi.org/10.1055/s-2001-10707
Cohen M, Petignat P (2014) The bright side of ascites in ovarian cancer. Cell Cycle 13:2319. https://doi.org/10.4161/cc.29951
Davis HA, Blalock JF (1939) Autologous and homologous transfusion of human ascitic fluid. J Clin Invest 18:219–224. https://doi.org/10.1172/JCI101037
Ito T, Hanafusa N, Iwase S et al (2015) Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int J Clin Oncol 20:623–628. https://doi.org/10.1007/s10147-014-0750-y
Maeda O, Ando T, Ishiguro K et al (2014) Safety of repeated cell-free and concentrated ascites reinfusion therapy for malignant ascites from gastrointestinal cancer. Mol Clin Oncol 2:1103–1106. https://doi.org/10.3892/mco.2014.335
Togami S, Hori S, Kamio M et al (2014) Clinical usefulness of concentrated ascites reinfusion therapy (CART) for gynecological cancer patients with refractory massive ascites due to cancerous peritonitis. Eur J Gynaecol Oncol 35:301–303
Ueda T, Maehara M, Takahashi Y et al (2012) Clinical significance of cell-free and concentrated ascites re-infusion therapy for advanced and recurrent gynecological cancer. Anticancer Res 32:2353–2357
Wang L, Okubo T, Shinsaka M et al (2015) Efficacy and safety of cell-free and concentrated ascites reinfusion therapy (CART) in gynecologic cancer patients with a large volume of ascites. J Obstet Gynaecol Res 41:1614–1620. https://doi.org/10.1111/jog.12763
Yamada Y, Yamaguchi A, Harada M et al (2017) Protein concentration of refractory ascites in cancer patients is reflected by the presence and severity of peritoneal and liver metastasis. Ther Apher Dial 21:263–269. https://doi.org/10.1111/1744-9987.12560
Yamaguchi H, Kitayama J, Emoto S et al (2015) Cell-free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S-1. Eur J Surg Oncol 41:875–880. https://doi.org/10.1016/j.ejso.2015.04.013
Hanada R, Yokomichi N, Kato C et al (2018) Efficacy and safety of reinfusion of concentrated ascitic fluid for malignant ascites: A concept-proof study. Support Care Cancer 26:1489–1497. https://doi.org/10.1007/s00520-017-3980-5
Yamada Y, Inui K, Hara Y et al (2019) Verification of serum albumin elevating effect of cell-free and concentrated ascites reinfusion therapy for ascites patients: a retrospective controlled cohort study. Sci Rep 9:10195. https://doi.org/10.1038/s41598-019-46774-9
Alexandre J, Gross-Goupil M, Falissard B et al (2003) Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe hematological toxicity following chemotherapy. Ann Oncol 14:36–41
Kloft C, Wallin J, Henningsson A et al (2006) Population pharmacokinetic-pharmacodynamic model for neutropenia with patient subgroup identification: comparison across anticancer drugs. Clin Cancer Res 12:5481–5490. https://doi.org/10.1158/1078-0432.CCR-06-0815
Joel SP, Shah R, Clark PI et al (1996) Predicting etoposide toxicity: relationship to organ function and protein binding. J Clin Oncol 14:257–267. https://doi.org/10.1200/JCO.1996.14.1.257
Hanafusa N, Isoai A, Ishihara T et al (2017) Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: post-marketing surveillance results. PLoS ONE 12:e0177303. https://doi.org/10.1371/journal.pone.0177303
Ito T, Hanafusa N, Fukui M et al (2014) Single center experience of cell-free and concentrated ascites reinfusion therapy in malignancy related ascites. Ther Apher Dial 18:87–92. https://doi.org/10.1111/1744-9987.12049
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Iwasa M, Ishihara T, Kato M et al (2019) Cell-free and concentrated ascites reinfusion therapy for refractory ascites in cirrhosis in post-marketing surveillance and the role of tolvaptan. Intern Med 58:3069–3075. https://doi.org/10.2169/internalmedicine.3091-19
Nagata Y, Kato K, Miyamoto T et al (2020) Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support Care Cancer 28:5861–5869. https://doi.org/10.1007/s00520-020-05401-4
Abe Y, Kobayashi H, Akizawa Y et al (2018) Possible application of ascites-infiltrating gamma-Delta T cells for adoptive immunotherapy. Anticancer Res 38:4327–4331. https://doi.org/10.21873/anticanres.12732
Jennifer JM, Henrik L, Berit JM et al (2012) International study of primary mucinous ovarian carcinomas managed at tertiary medical centers. Int J Gynecol Cancer 28:915–924. https://doi.org/10.1097/IGC.0000000000001263
Acknowledgements
We would like to thank the staff of all of the centers that participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ishitani, K., Isoai, A., Ito, T. et al. Clinical usefulness of cell-free and concentrated ascites reinfusion therapy (CART) in combination with chemotherapy for malignant ascites: a post-marketing surveillance study. Int J Clin Oncol 26, 1130–1138 (2021). https://doi.org/10.1007/s10147-021-01883-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01883-2