Abstract
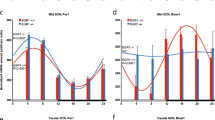
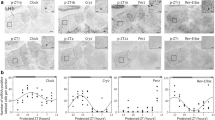
Photic and non-photic inputs are reported to affect clock gene expressions and behavioral activities in the SCN. However, it is not known whether dopaminergic input mediates these regulatory effects on clock genes. The present study examined the molecular effects of dopamine D1 agonist on Per1, Per2, CLOCK, and Bmal1 expressions in the SCN and its effect on behavioral activities to determine the role of dopamine D1 receptor in regulation of these gene expressions and behavioral activities in adult male Wistar rats. To examine the molecular effects of dopamine D1 agonist day and night, we injected 20 mg/kg SKF38393 to the first group of rats at 6 a.m. and the second group at 6 p.m. We also injected saline to the third and fourth groups of rats at 6 a.m. and 6 p.m. as control groups. All rats were sacrificed 2 h following the injections. The real-time PCR technique was used to evaluate the clock gene expression. In addition, to examine the effects of dopamine D1 agonists on behavioral activities, we injected 20 mg/kg SKF38393 to SKF receiving group and saline to control group. The behavioral activities of the rats were monitored on the running wheel for 21 days, 1 week following the injections. SKF injections increased the Per2 and CLOCK expressions in the daytime and significantly decreased the Per1 and Bmal1 expressions. However, at night, SKF injections increased only Per2 expressions significantly and decreased the Per1, CLOCK, and Bmal1 genes expressions. Both saline receiving groups showed that all gene expressions were significantly higher except Per2 during nighttime. SKF injection increased the running wheel activity during nighttime significantly. Based on the obtained result, clock gene expression and behavioral activities in adult male Wistar rats may be altered or monitored by administration of exogenous dopamine.







Similar content being viewed by others
Availability of Data and Materials
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
References
Abdul F, Sreenivas N, Kommu JV, Banerjee M, Berk M, Maes M, Leboyer M, Debnath M (2021) Disruption of circadian rhythm and risk of autism spectrum disorder: role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways. Rev Neurosci
Ashton A, Jagannath A (2020) Disrupted sleep and circadian rhythms in schizophrenia and their interaction with dopamine signaling. Front Neurosci 14
Astiz M, Heyde I, Oster H (2019) Mechanisms of communication in the mammalian circadian timing system. Int J Mol Sci 20:343
Barca‐Mayo O, Boender AJ, Armirotti A, De Pietri Tonelli D (2020) Deletion of astrocytic BMAL1 results in metabolic imbalance and shorter lifespan in mice. Glia 68:1131–1147
Begemann K, Neumann AM, Oster H (2020) Regulation and function of extra‐SCN circadian oscillators in the brain. Acta Physiologica 229:e13446
Besharse JC, Zhuang M, Freeman K, Fogerty J (2004) Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur J Neurosci 20:167–174
Cheng AH, Cheng HY (2021) Genesis of the master circadian pacemaker in mice. Front in Neurosci 15
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Cox KH, Takahashi JS (2019) Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol 63:R93–R102
Csép K (2021) Transcription factors of the core feedback loop in the molecular circadian clock machinery: internal timekeeping and beyond. Acta Marisiensis-Seria Medica 67
Dardente H, Wyse CA, Lincoln GA, Wagner GC, Hazlerigg DG (2016) Effects of photoperiod extension on clock gene and neuropeptide RNA expression in the SCN of the Soay sheep. PloS One 11:e0159201
Fujieda H, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM (1999) Expression of mt1 melatonin receptor in rat retina: evidence for multiple cell targets for melatonin. Neuroscience 93:793–799
Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM (2000) Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci 17:63–70
Grippo RM, Güler AD (2019) Focus: clocks and cycles: dopamine signaling in circadian photoentrainment: consequences of desynchrony. Yale J Biol Med 92:271
Grippo RM, Purohit AM, Zhang Q, Zweifel LS, Güler AD (2017) Direct midbrain dopamine input to the suprachiasmatic nucleus accelerates circadian entrainment. Curr Biol 27:2465–2475 e3
Haque SN, Booreddy SR, Welsh DK (2019) Focus: clocks and cycles: effects of bmal1 manipulation on the brain’s master circadian clock and behavior. Yale J Biol Med 92:251
Hiragaki S, Baba K, Coulson E, Kunst S, Spessert R, Tosini G (2014) Melatonin signaling modulates clock genes expression in the mouse retina. PloS One 9:e106819
Hou Y, Liu L, Chen X, Li Q, Li J (2020) Association between circadian disruption and diseases: a narrative review. Life Sci p 118512
Imbesi M, Sevim Yildiz A, Arslan D, Sharma R, Manev H, Uz T (2009) Dopamine receptor-mediated regulation of neuronal clock gene expression. Neuroscience 158:537–544
Itzhacki J, Clesse D, Goumon Y, Van Someren EJ, Mendoza J (2018) Light rescues circadian behavior and brain dopamine abnormalities in diurnal rodents exposed to a winter-like photoperiod. Brain Struct Funct 223:2641–2652
Jiang N, Wang Z, Cao J, Dong Y, Chen Y (2017) Effect of monochromatic light on circadian rhythmic expression of clock genes in the hypothalamus of chick. J Photochem Photobiol B 173:476–484
Li H, Song S, Wang Y, Huang C, Zhang F, Liu J, Hong J-S (2019) Low-grade inflammation aggravates rotenone neurotoxicity and disrupts circadian clock gene expression in rats. Neurotox Res 35:421–431
Luo S, Ezrokhi M, Cominos N, Tsai T-H, Stoelzel CR, Trubitsyna Y, Cincotta AH (2021) Experimental dopaminergic neuron lesion at the area of the biological clock pacemaker, suprachiasmatic nuclei (SCN) induces metabolic syndrome in rats. Diabetol Metab Syndr 13:1–17
Manzanares G, Brito-da-Silva G, Gandra PG (2019) Voluntary wheel running: patterns and physiological effects in mice. Brazil J Med Biol Res 52
Mendoza J (2019) Circadian insights into the biology of depression: symptoms, treatments and animal models. Behav Brain Res 376:112186
Mieda M (2020) The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci Res 156:24–31
Mouland JW, Martial FP, Lucas RJ, Brown TM (2021) Modulations in irradiance directed at melanopsin, but not cone photoreceptors, reliably alter electrophysiological activity in the suprachiasmatic nucleus and circadian behaviour in mice. J Pineal Res 70:e12735
Ono D, Honma K-I, Honma S (2015) Circadian and ultradian rhythms of clock gene expression in the suprachiasmatic nucleus of freely moving mice. Sci Rep 5:1–10
Oyegbami O, Collins HM, Pardon M-C, Ebling FJP, Heery DM, Moran PM (2017) Abnormal clock gene expression and locomotor activity rhythms in two month-old female APPSwe/PS1dE9 mice. Curr Alzheimer Res 14:850–860
Papazyan R, Zhang Y, Lazar MA (2016) Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat Struct Mol Biol 23:1045–1052
Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F (2003) Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 120:745–755
Ruan GX, Allen GC, Yamazaki S, McMahon DG (2008) An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol 6:e249
Sabbar M, Dkhissi-Benyahya O, Benazzouz A, Lakhdar-Ghazal N (2017) Circadian clock protein content and daily rhythm of locomotor activity are altered after chronic exposure to lead in rat. Front Behav Neurosci 11:178
Shearman LP, Weaver DR (2001) Distinct pharmacological mechanisms leading to c-fos gene expression in the fetal suprachiasmatic nucleus. J Biol Rhythms 16:531–540
Shimomura H, Moriya T, Sudo M, Wakamatsu H, Akiyama M, Miyake Y, Shibata S (2001) Differential daily expression of Per1 and Per2 mRNA in the suprachiasmatic nucleus of fetal and early postnatal mice. Eur J Neurosci 13:687–693
Smolensky MH, Hermida RC, Geng Y-J (2021) Chronotherapy of cardiac and vascular disease: timing medications to circadian rhythms to optimize treatment effects and outcomes. Curr Opin Pharmacol 57:41–48
Stoynev AG, Ikonomov OC, Stoynev NA (2021) Suprachiasmatic hypothalamic nuclei (SCN) in regulation of homeostasis: a role beyond circadian control?. Biol Rhythm Res p 1–18
Taymans J-M, Kia HK, Claes R, Cruz C, Leysen J, Langlois X (2004) Dopamine receptor-mediated regulation of RGS2 and RGS4 mRNA differentially depends on ascending dopamine projections and time. Eur J Neurosci 19:2249–2260
Tucci V, Isles AR, Kelsey G, Ferguson-Smith AC, Bartolomei MS, Benvenisty N, Bourc’his D, Charalambous M, Dulac C, Feil R (2019) Genomic imprinting and physiological processes in mammals. Cell 176:952–965
Vriend J, Reiter RJ (2015) Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 58:1–11
Wang P, Gao X, Zhao F, Gao Y, Wang K, Tian JS, Li Z, Qin XM (2021) Study of the neurotransmitter changes adjusted by circadian rhythm in depression based on liver transcriptomics and correlation analysis. ACS Chem Neurosci
Xie Y, Tang Q, Chen G, Xie M, Shaoling Yu, Zhao J, Chen L (2019) New insights into the circadian rhythm and its related diseases. Front Physiol 10:682
Xu Y, Pan D (2018) Interpretation of the Nobel Prize in Physiology or Medicine 2017, Science China. Life Sci 61:131–134
Yamada H, Kuroki T, Nakahara T, Hashimoto K, Tsutsumi T, Hirano M, Maeda H (2007) The dopamine D1 receptor agonist, but not the D2 receptor agonist, induces gene expression of Homer 1a in rat striatum and nucleus accumbens. Brain Res 1131:88–96
Yan L, Okamura H (2002) Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci 15:1153–1162
Acknowledgements
The authors are grateful to the Neuroscience Research Center of Iran University of Medical Sciences for the supply of laboratory and technical issues.
Funding
This work was supported by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996) and Hearing Disorders Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, and Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Abbas Haghparast was responsible for the study concept and design. Somaye Mesgar contributed to the acquisition of behavioral and molecular data. Abbas Aliaghaei, Somaye Mesgar, and Abolfazl Torabi assisted with data analysis and interpretation of findings. Abbas Haghparast and Somaye Mesgar drafted the manuscript. Abbas Haghparast, Seyed Behnamedin Jameie, Siavash Parvardeh, and Abbas Aliaghaei provided critical revision of the manuscript for important intellectual content. All authors critically reviewed the content and approved the final version for publication.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All animals’ experiments were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996) and were approved by the Research and Ethics Committee of School of Medicine, Shahid Beheshti University of Medical Sciences (IR.SBMU.SM.REC. 1394.162), Tehran, Iran.
Consent for Publication
The authors agree to publish this article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mesgar, S., Jameie, S.B., Aliaghaei, A. et al. Dopamine D1 Receptor-Mediated Regulation of Per1, Per2, CLOCK, and BMAL1 Expression in the Suprachiasmatic Nucleus in Adult Male Rats. J Mol Neurosci 72, 618–625 (2022). https://doi.org/10.1007/s12031-021-01923-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01923-6