Abstract
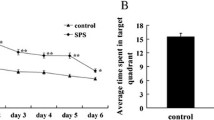
The goal of this study was to further elucidate the molecular mechanisms of post-traumatic stress disorder (PTSD) pathogenesis and to provide experimental evidence for new drug targets for effective PTSD treatment. Expression changes of IRE1α, ASK1, and other downstream molecules of the IRE1α-ASK1 endoplasmic reticulum stress (ERS) signaling pathway were investigated. JNK, P38, CHOP, Bcl-2, and Bax were analyzed at both protein and mRNA levels of dorsal raphe nucleus (DRN) neurons of PTSD rats. The rat PTSD model was established via the single-prolonged stress (SPS) method. Animals were randomly divided into five groups: a normal control group, a 1-day SPS group, a 4-days SPS group, a 7-day SPS group, and a 14-day SPS group. Spatial memory and learning ability of rats were evaluated subsequent to SPS using the Morris water maze test. Changes of IRE1α expression in the control and SPS groups were detected via immunohistochemistry (IHC). Protein and mRNA expressions of IRE1α, ASK1, JNK, P38, CHOP, Bcl-2, and Bax in the control and SPS groups were detected via Western blot and RT-PCR, respectively. The Morris water maze test revealed significantly longer average escape latencies in all SPS groups compared to the control group. In the spatial probe test, the percentage of time spent in the target quadrant was significantly lower in the SPS groups compared to control. IHC revealed increased positive expression of IRE1α subsequent to SPS challenge, reaching maximal levels on days four and seven (P < 0.01), while significantly decreasing on day 14 (P < 0.01). Western blot and RT-PCR revealed that protein and mRNA expressions of IRE1α, ASK1, JNK, CHOP, and P38 were significantly increased compared to control, peaking on days one, four, and seven post-SPS before returning to previous levels. Compared to control, expressions of Bcl-2 and Bax presented an initial increasing tendency followed by a decrease. A peak of Bcl-2 expression appeared early on day one following SPS, then decreased to a steady level. Bax expression in the SPS groups remained constant during early stages after SPS (days one to three) compared to control; however, expression significantly increased on day four and maintained a high level. In summary, 1) SPS challenge significantly activated the IRE1α-ASK1-JNK and IRE1α-ASK1-P38 apoptosis-signaling pathways in DRN neurons of PTSD rats. This resulted in a cascade of downstream reactions and ultimately apoptosis of DRN neurons. 2) Increased expression of apoptosis-associated molecules Bcl-2 and Bax in DRN neurons following SPS challenge was revealed as a central mechanism, inducing apoptosis of DRN neurons in PTSD rats.









Similar content being viewed by others
References
Bartlett JD, Luethy JD, Carlson SG et al (1992) Calcium ionophore A23187 induces expression of the growth arrest and DNA damage inducible CCAAT/enhancer-binding protein (C/EBP)-related gene, gadd153.Ca2+ increases transcriptional activity and mRNA stability. J Biol Chem 267:20465–20470
Bremner JD, Elzinga B, Schmahl C et al (2008) Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res 167:171–186
Brewer JW et al (2014) Regulatory crosstalk within the mammalian unfolded protein response. Cell Mol Life Sci 71:1067–1079
Chen Q, Yu K, Holbrook NJ et al (1992) Activation of the growth arrest and DNA damage-inducible gene gadd 153 by nephrotoxic cysteine conjugates and dithiothreitol. J Biol Chem 267(12):8207–8212
Cox RF, Meller E, Waszczak BL (1993) Electrophysiological evidence for a large receptor reserve for inhibition of dorsal raphe neuronal firing by 5-HT1A agonists. Synapse 14(4):297–304
Ding J, Han F, Shi Y (2010) Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. J Psychiatr Res 44(1):48–55
Eldadah B, Faden A (2000) Caspase pathways, neuronal apoptosis and CNS injury. J Neurotrauma 17(10):811–829
Fleischer A, Rebollo A, Ayllon V (2003) BH3-only proteins: the lords of death. Arch Immunol Ther Exp 51:9–17
Galehdar Z, Swan P, Fuerth B et al (2010) Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci 30(50):16938–16948
George SA, Rodriguez-Santiago M, Riley J et al (2015) Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav Brain Res 286:256–264
Gupta S, Deepti A, Deegan S et al (2010) HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol 8(7):e1000410
Han J, Lee JD, Bibbs L et al (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265(5173):808–811
Ichijo H, Nishida E, Irie K et al (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275(5296):90–94
Johnson GG, White MC, Grimaldi M (2011) Stressed to death: targeting endoplasmic reticulum stress response induced apoptosis in gliomas. Curr Pharm Des 17(3):284–292
Katayama T, Imaizumi K, Honda A et al (2001) Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer’s disease-linked presenilin-1 mutations. J Biol Chem 276:43446–43454
Kim DS, Kim JH, Lee GH et al (2010) p38 mitogen-activated protein kinase is involved in endoplasmic reticulum stress-induced cell death and autophagy in human gingival fibroblasts. Biol Pharm Bull 33(4):545–549
Li X, Han F, Liu D et al (2010) Changes of Bax, Bcl-2 and apoptosis in hippocampus in the rat model of post-traumatic stress disorder. Neurol Res 32:579–586
Li S, Shi Y, Kirouac GJ (2014) The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front Neuroanat 8:136
Li X, Han F, Shi Y (2015) IRE1α-XBP1 pathway is activated upon induction of single-prolonged stress in rat neurons of the medial prefrontal cortex. J Mol Neurosci 57(1):63–72
Liu J, Lin A (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15(1):36–42
Liu D, Xiao B, Han F et al (2012) Single-prolonged stress induces apoptosis in dorsal raphe nucleus in the rat model of posttraumatic stress disorder. BMC Psychiatry 12:211
Luo FF, Han F, Shi YX (2011) Changes in 5-HT1A receptor in the dorsal raphe nucleus in a rat model of post-traumatic stress disorder. Mol Med Rep 4(5):843–847
Malhi H, Kaufman RJ (2011) Endoplasmic reticulum stress in liver disease. J Hepatol 54(4):795–809
Malhotra JD, Kaufman RJ (2007) The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18(6):716–731
Mccullough KD, Martindale JL, Klotz LO et al (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259
Oyadomari S, Koizumi A, Takeda K et al (2002) Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109:525–532
Pollice R, Bianchini V, Roncone R et al (2012) Psychological distress and post-traumatic stress disorder (PTSD) in young survivors of L’Aquila earthquake. Riv Psichiatr 47(1):59–64
Ravanti L, Toriseva M, Penttinen R et al (2001) Expression of human collagenase-3 (MMP-13) by fetal skin fibroblasts is induced by transforming growth factor beta via p38 mitogen-activated protein kinase. FASEB J 15(6):1098–1100
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Skommer J, Wlodkowic D, Deptala A (2007) Larger than life: mitochondria and the Bcl-2 family. Leuk Res 31(3):277–286
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190
Tobiume K, Saitoh M, Ichijo H (2002) Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol 191(1):95–104
van Praag HM (2004) The cognitive paradox in posttraumatic stress disorder: a hypothesis. Prog Neuro-Psychopharmacol Biol Psychiatry 28(6):923–935
Ventura JJ, Hübner A, Zhang C et al (2006) Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell 21(5):701–710
Wan J, Liu D, Zhang J et al (2016) Single-prolonged stress induce different change in the cell organelle of the hippocampal cells: a study of ultrastructure. Acta Histochem 118(1):10–19
Wang W, Guo Y, Xu M et al (2011) Development of diabetes in lean Ncb5or-null mice is associated with manifestations of endoplasmic reticulum and oxidative stress in beta cells. Biochim Biophys Acta 1812(11):1532–1541
Wen Y, Li B, Han F et al (2012) Dysfunction of calcium/calmodulin/CaM kinase IIα cascades in the medial prefrontal cortex in post-traumatic stress disorder. Mol Med Report 6(5):1140–1144
Wu ZM, Zheng CH, Zhu ZH et al (2016) SiRNA-mediated serotonin transporter knockdown in the dorsal raphe nucleus rescues single prolonged stress-induced hippocampal autophagy in rats. J Neurol Sci 360:133–140
Xie J, Han F, Shi Y (2014) The unfolded protein response is triggered in rat neurons of the dorsal raphe nucleus after single-prolonged stress. Neurochem Res 39(4):741–747
Xie J, Han F, Shi Y (2015) Single-prolonged stress activates the transcription factor ATF6α branch of the unfolded protein response in rat neurons of dorsal raphe nucleus. Mol Cell Biochem 399(1–2):209–216
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279:45495–45502
Yoshida H, Haze K, Yanagi H et al (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273(50):33741–33749
Zhang C, Kawauchi J, Adachi MT et al (2001) Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem Biophys Res Commun 289(3):718–724
Zhang Y, Liu W, Zhou Y, Ma C, Li S, Cong B (2014) Endoplasmic reticulum stress is involved in restraint stress-induced hippocampal apoptosis and cognitive impairments in rats. Physiol Behav 131:41–48
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81571324), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20132104110021), and Shenyang Science and Technology Project (No. F16-205-1-35).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yuxiu Shi is the first corresponding author of this paper.
Rights and permissions
About this article
Cite this article
Kong, F., Han, F., Xu, Y. et al. Molecular Mechanisms of IRE1α-ASK1 Pathway Reactions to Unfolded Protein Response in DRN Neurons of Post-Traumatic Stress Disorder Rats. J Mol Neurosci 61, 531–541 (2017). https://doi.org/10.1007/s12031-017-0895-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0895-z