Abstract
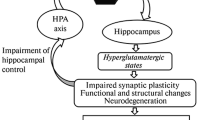
Although dexamethasone (DEX), a synthetic glucocorticoid receptor (GR) analog with profound effects on energy metabolism, immune system, and hypothalamic-pituitary-adrenal axis, is widely used therapeutically, its impact on the brain is poorly understood. The aim of the present study was to explore the effect of repeated low-dose DEX administration on the activity and expression of the ectonucleotidase enzymes which hydrolyze and therefore control extracellular ATP and adenosine concentrations in the synaptic cleft. Ectonucleotidases tested were ectonucleoside triphosphate diphosphohydrolase 1–3 (NTPDase1–3) and ecto-5′-nucleotidase (eN), whereas the effects were evaluated in two brain areas that show different sensitivity to glucocorticoid action, hippocampus, and cerebral cortex. In the hippocampus, but not in cerebral cortex, modest level of neurodegenerative changes as well as increase in ATP, ADP, and AMP hydrolysis and upregulation of NTPDase1 and eN mRNA expression ensued under the influence of DEX. The observed pattern of ectonucleotidase activation, which creates tissue volume with enhanced capacity for adenosine formation, is the hallmark of the response after different insults to the brain.





Similar content being viewed by others
References
Abraham IM, Harkany T, Horvath KM, Luiten PG (2001) Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection. J Neuroendocrinol 13(9):749–760
Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, et al (2000) Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J 14:779–790
Barnes PJ (2005) Molecular mechanisms and cellular effects of glucocorticosteroids. Immunol Alergy Clin North Am 25:451–468
Bavaresco L, Bernardi A, Braganhol E, Wink MR, Battastini AM (2007) Dexamethasone inhibits proliferation and stimulates ecto-5′-nucleotidase/CD73 activity in C6 rat glioma cell line. J Neuro oncol 84:1–8
Belcher SM, Zsarnovsky A, Crawford PA, Hemani H, Spurling L, Kirley TL (2006) Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep wake behaviors. Neuroscience 137:1331–1346
Bjelobaba I, Lavrnja I, Parabucki A, Stojkov D, Stojiljkovic M, Pekovic S, Nedeljkovic N (2010) The cortical stab injury induces beading of fibers expressing ecto-nucleoside triphosphate diphosphohydrolase3. Neuroscience 170:107–116
Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H (1998) Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci 18:4891–4900
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Cunha RA (2005) Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blocade. Purinergic Signal 1(2):111–113
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301
Drakulić D, Veličković N, Stanojlović M, Grković I, Mitrović N, Lavrnja I, Horvat A (2013) Low-dose dexamethasone treatment promotes the pro-survival signalling pathway in the adult rat prefrontal cortex. J Neuroendocrinol 25(7):605–616
Dunwiddle TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55
Elkouby A, Ledig M, Mandel P (1982) Effect of hydrocortisone and thyroxine on ATPase activities of neuronal and glial cell lines in culture. Neurochem Res 7:387–397
Fuxe K, Wiksyrom AC, Gustafsson JA (1985) Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinol 117:1803–1812
GeneCards: ENTPD1; HGNC 3363; Entrez gene: 953; Ensembl: ENSG0000138185; OMIM 601752
GeneCards: NT5E; HGNC 8021; Entrez gene: 4907; Ensembl: ENSG00000135318; OMIM 129190
Haynes LE, Griffiths MR, Hyde RE, Barber DJ, Mitchell IJ (2001) Dexamethasone induces limited apoptosis and extensive sublethal damage to specific subregions of the striatum and hippocampus: implications for mood disorders. Neuroscience 104(1):57–69
Horvat A, Stanojevic I, Drakulic D, Velickovic N, Petrovic S, Milosević M (2010) Effect of acute stress on NTPDase and 5′-nucleotidase activities in brain synaptosomes in different stages of development. Int J Dev Neurosci 28(2):175–182
Jacobson KA, Hoffmann C, Cattebeni F, Abbracchio MP (1999) Adenosine-induced cell death: evidence for receptor-mediated signaling. Apoptosis 4:197–211
James G, Butt AM (2002) P2Y and P2X purinoreceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol 447:247–260
Karssen AM, Meijer OC, Berry A, Sanjuan Pinol R, de Kloet ER (2005) Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinol 146:5587–5595
Kegel B, Braun N, Heine P, Maliszewski CR, Zimmermann H (1997) An ecto-ATPase and an ecto-ATP diphosphohydrolase are expressed in rat brain. Neuropharmacology 36:1189–1200
Kino T (2007) Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res 39(6):420–424
Kukulski F, Komoszynski M (2003) Purification and characterization of NTPDase 1 (ecto-apyrase) and NTPDase 2 (ecto-ATPase) from porcine brain cortex synaptosomes. Eur J Biochem 270:3447–3454
Langer D, Hammer K, Koszalka P, Schrade J, Robson S, Zimmermann H (2008) Ditribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res 334:199–217
Markwell MA, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87(1):206–210
McEwen BS, de Kloet R, Wallach G (1976) Interactions in vivo and in vitro of corticoids and progesterone with cell nuclei and soluble macromolecules from rat brain regions and pituitary. Brain Res 105:129–136
Nedeljkovic N, Djordjevic V, Horvat A, Nikezic G, Kanazir D (2000) Effect of steroid hormone deprivation on the expression of ecto-ATPase in distinct brain regions of female rats. Physiol Res 49:419–426
Nedeljkovic N, Bjelobaba I, Subasic S, Lavrnja I, Pekovic S, Stojkov D, Vjestica A, Rakic L, Stojiljkovic M (2006) Up-regulation of ectonucleotidase activity after cortical stab injury in rats. Cell Biol Int 30:541–546
Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E (2010) The human glucocorticoid receptor: molecular basis of biologic function. Steroids 75:1–12
Reul JM, De Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505–2511
Reul JM, van den Bosch JR, De Kloet ER (1987) Relative occupation of type-I and type-II corticosteroid receptors in the rat brain following stress and dexamethasone treatment: functional implications. J Endocrinol 115:459–467
Reul JM, Gesing A, Droste S, Stec IS, Weber A, Bachmann C, Bilang-Bleuel A, Holsboer F, Linthorst AC (2000) The brain mineralcorticoid receptor: greedy for ligand, mysterious in function. Eur J Pharmacol 405:235–249
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure functions relations and pathophysiological signifficance. Purinergic Signal 2:409–430
Saucedo E, Pereira R, Barbosa G, Battisti V, Leal CA, Fleck J, Santos RC, Morsch VM, Schetinger MR, Leal DB (2010) Enzymes that hydrolyze adenine nucleotides in lymphocytes and platelets of immunosuppressed rats. Biomed Pharmacother 64(6):437–440
Schäcke H, Döcke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23–43
Smith TM, Kirley TL (1998) Cloning, sequencing and expression of a human brain ecto-apyrase. Biochim Biophys Acta 1386:65–78
Stone TW (2005) Adenosine, neurodegeneration and neuroprotection. Neurol Res 27:161–168
van Bogaert T, De Bosscher K, Libert C (2010) Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev 21:275–286
van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C (2000) Use of oral corticosteroids in the United Kingdom. QJM 93:105–111
Vollmayer P, Koch M, Braun N, Heine P, Servos J, Israr E, Kegel B, Zimmermann H (2001) Multiple ecto-nucleotidase in PC12 cells: identification and cellular distribution after heterologous expression. J Neurochem 78:1019–1028
Wink MR, Braganhol E, Tamajusuku AS, Lenz G, Zerbini LF, Libermann TA, Sévigny J, Battastini AM, Robson SC (2006) Nucleoside triphosphate diphosphohydrolase-2(NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience 138:421–432
Zimmermann H, Zebisch M, Strater N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502
Acknowledgments
The authors gratefully appreciate Dr. Irena Lavrnja for the assistance with Fluoro-Jade B staining. This work was supported by the Serbian Ministry of Education and Science, Project Nos. 173044 and 41014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drakulić, D., Stanojlović, M., Nedeljković, N. et al. Upregulation of Nucleoside Triphosphate Diphosphohydrolase-1 and Ecto-5′-Nucleotidase in Rat Hippocampus after Repeated Low-Dose Dexamethasone Administration. J Mol Neurosci 55, 959–967 (2015). https://doi.org/10.1007/s12031-014-0452-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0452-y