Abstract
Introduction
Hepatocellular carcinoma is a lethal disease and there has been a debate regarding the first-line treatment of its advanced and unresectable form. Observational studies have explored atezolizumab plus bevacizumab versus lenvatinib, yielding mixed results. This systematic review and meta-analysis aim to compare efficacy and safety of both treatment arms.
Methods
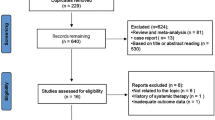
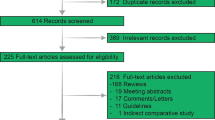
A systematic literature review was conducted in accordance with PRISMA guidelines. Randomized control trials, cohort studies, or case–control that included patients above age 60 with unresectable hepatocellular carcinoma confirmed by radiological imaging were included. At least one of the outcomes: overall survival (OS), progression-free survival (PFS), objective response rate (ORR), duration of response, or adverse events was included in the selected studies.
Results
Ten cohorts were included in the analysis with a total of 6493 patients. Nine of the included studies had patients with advanced HCC (BCLC-C) or intermediate HCC (BCLC-B) and 1 study included patients with all three stages (BCLC-A, BCLC-B, and BCLC-C). Of these patients, 2524 patients received atezolizumab plus bevacizumab (A + B) combination while 3969 received lenvatinib. The overall survival was better statistically in the A + B group then the lenvatinib group (MD: − 5.06; 95% CI: − 7.79 to − 2.33; p = 0.0003, I2 = 0%). The progression-free survival was significantly improved in A + B arm as well group (MD: − 4.96; 95% CI: − 7.67 to − 2.26; I2 = 0%, p = 0. 0003). There was no significant difference in objective response rate, disease control rate, and frequency of adverse events in either of the group.
Conclusion
Our study concluded that combination therapy with atezolizumab plus bevacizumab could increase the survival duration without affecting the disease course. Moreover, while the severity of adverse events was greater in the A + B group, their frequency was comparable to the lenvatinib group.






Similar content being viewed by others
Data Availability
The datasets associated with this study can be obtained upon reasonable request from the corresponding author.
References
Cancer Statistics. CA: A Cancer Journal for Clinicians Materials and methods data sources. 2022;8. https://doi.org/10.3322/caac.21708.
Systemic treatment for advanced hepatocellular carcinoma - UpToDate. Accessed 01 Dec 2023. Available: https://www.uptodate.com/contents/systemic-treatment-for-advanced-hepatocellular-carcinoma
Llovet JM, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7(1):1–28. https://doi.org/10.1038/s41572-020-00240-3.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi.org/10.1056/NEJMOA0708857/SUPPL_FILE/NEJM_LLOVET_378SA1.PDF.
Kudo M, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. https://doi.org/10.1016/S0140-6736(18)30207-1.
Llovet JM, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–72. https://doi.org/10.1038/S41571-021-00573-2.
Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. https://doi.org/10.1056/NEJMOA1915745.
Niizeki T, et al. Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a propensity score matching analysis. Target Oncol. 2022;17(6):643–53. https://doi.org/10.1007/S11523-022-00921-X.
Persano M, et al. Clinical outcomes with atezolizumab plus bevacizumab or lenvatinib in patients with hepatocellular carcinoma: a multicenter real-world study. J Cancer Res Clin Oncol. 2023;149(9):5591–602. https://doi.org/10.1007/S00432-022-04512-1.
Rimini M, et al. Survival outcomes from atezolizumab plus bevacizumab versus Lenvatinib in Child Pugh B unresectable hepatocellular carcinoma patients. J Cancer Res Clin Oncol. 2023;149(10):7565–77. https://doi.org/10.1007/S00432-023-04678-2.
Su CW, et al. Similar efficacy and safety between lenvatinib versus atezolizumab plus bevacizumab as the first-line treatment for unresectable hepatocellular carcinoma. Cancer Med. 2023;12(6):7077–89. https://doi.org/10.1002/CAM4.5506.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2010. https://doi.org/10.1016/j.ijsu.2010.02.007.
Ottawa Hospital Research Institute. Accessed on 01 Dec 2023. Available: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Rimini M, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open. 2022;7(6). https://doi.org/10.1016/J.ESMOOP.2022.100591.
Kim BK, et al. Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel). 2022;14(7). https://doi.org/10.3390/CANCERS14071747.
Hiraoka A, et al. Does first-line treatment have prognostic impact for unresectable HCC?-Atezolizumab plus bevacizumab versus lenvatinib. Cancer Med. 2023;12(1):325–34. https://doi.org/10.1002/CAM4.4854.
Casadei-Gardini A, et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a large real-life worldwide population. Eur J Cancer. 2023;180:9–20. https://doi.org/10.1016/J.EJCA.2022.11.017.
Sasaki, R et al. Evaluating the role of hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging in predicting treatment impact of lenvatinib and atezolizumab plus bevacizumab on unresectable hepatocellular carcinoma. Cancers (Basel). 2022;14(3). https://doi.org/10.3390/CANCERS14030827.
Maesaka K, et al. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res. 2022;52(7):630–40. https://doi.org/10.1111/HEPR.13771.
Salem R, et al. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: results from the IMbrave150 trial. Cancer Med. 2021;10(16):5437. https://doi.org/10.1002/CAM4.4090.
Galle PR, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. https://doi.org/10.1016/S1470-2045(21)00151-0.
Du S, Cao K, Wang Z, Lin D. Clinical efficacy and safety of atezolizumab plus bevacizumab versus lenvatinib in the treatment of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Medicine. 2023;102(23):E33852. https://doi.org/10.1097/MD.0000000000033852.
Liu J, Yang L, Wei S, Li J, Yi P. Efficacy and safety of atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2023;149(17):16191–201. https://doi.org/10.1007/S00432-023-05342-5.
Kudo M, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181. https://doi.org/10.1159/000514174.
Cheng AL, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. https://doi.org/10.1016/J.JHEP.2021.11.030.
Ueshima K, et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: a multicenter study. Cancers (Basel). 2019;11(7). https://doi.org/10.3390/CANCERS11070952.
Hatanaka T, et al. Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: a multicenter retrospective study. Hepatol Res. 2020;50(3):382–95. https://doi.org/10.1111/HEPR.13460.
Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10(6):559–68. https://doi.org/10.1016/S1470-2045(09)70112-3.
Pomej K, et al. Vascular complications in patients with hepatocellular carcinoma treated with sorafenib. Cancers (Basel). 2020;12(10):1–14. https://doi.org/10.3390/CANCERS12102961.
Zhang X, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7(1):1–10. https://doi.org/10.1186/S40425-019-0808-5/FIGURES/3.
Author information
Authors and Affiliations
Contributions
Changez MIK made substantial contributions to the conception or design of the study and drafted the manuscript. Khan M, Tahir MF, Mohsin M drafted the manuscript. Changez MIK, Khan M and Ahmed AH contributed in analyses of the data. Hussain AF, Saqib V, and Molani MK participated in literature search and data extraction. Uzair M revised the article critically for intellectual content. Khalid S supervised the project, made contributions to the conception and design of the study and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
Authors have nothing to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Changez, M., Khan, M., Uzair, M. et al. Efficacy of Atezolizumab Plus Bevacizumab Versus Lenvatinib in Patients with Unresectable Hepatocellular Carcinoma: a Meta-analysis. J Gastrointest Canc 55, 467–481 (2024). https://doi.org/10.1007/s12029-023-00999-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-023-00999-0