Abstract
Purpose
Although second-line treatments improve survival compared to best supportive care in patients with advanced gastric cancer with disease progression on first-line therapy, prognosis remains poor. A systematic review and meta-analysis were conducted to quantify the efficacy of second-or-later line systemic therapies in this target population.
Methods
A systematic literature review (January 1, 2000 to July 6, 2021) of Embase, MEDLINE, and CENTRAL with additional searches of 2019–2021 annual ASCO and ESMO conferences was conducted to identify studies in the target population. A random-effects meta-analysis was performed among studies involving chemotherapies and targeted therapies relevant in treatment guidelines and HTA activities. Outcomes of interest were objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) presented as Kaplan–Meier data. Randomized controlled trials reporting any of the outcomes of interest were included. For OS and PFS, individual patient-level data were reconstructed from published Kaplan–Meier curves.
Results
Forty-four trials were eligible for the analysis. Pooled ORR (42 trials; 77 treatment arms; 7256 participants) was 15.0% (95% confidence interval (CI) 12.7–17.5%). Median OS from the pooled analysis (34 trials; 64 treatment arms; 60,350 person-months) was 7.9 months (95% CI 7.4–8.5). Median PFS from the pooled analysis (32 trials; 61 treatment arms; 28,860 person-months) was 3.5 months (95% CI 3.2–3.7).
Conclusion
Our study confirms poor prognosis among patients with advanced gastric cancer, following disease progression on first-line therapy. Despite the approved, recommended, and experimental systemic treatments available, there is still an unmet need for novel interventions for this indication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With over a million new cases per year worldwide, gastric cancer is one of the leading causes of cancer-related mortality [1]. Despite declining incidence rates, gastric cancer is the most frequently diagnosed cancer in men in many South Central Asian countries, with the highest incidence rates in Eastern Asia and Eastern Europe [1]. Five-year survival rates range from 23 to 45% in patients with the locally advanced disease who receive chemotherapy before and after surgery [2,3,4], whereas that of distant metastatic disease is only 6% [5]. With the exception of Asian countries, where routine screening is performed, patients are often diagnosed at a late stage, where surgery is not an option, or with distant metastasis. For those who are diagnosed at earlier stages, surgery is a possible cure; however, the majority will still experience disease progression following resection [6].
Several single-agent and combination therapies have been recommended for advanced gastric cancer, including chemotherapy, chemoradiation, immunotherapy, and targeted therapy, depending on the disease characteristics [6, 7]. Specifically, doublet or triplet chemotherapy regimens containing platinum, fluoropyrimidine, or taxanes are recommended for first-line treatment of advanced gastric cancer [6]. In this setting, patients with HER-2 overexpression positive tumors would benefit from trastuzumab, a HER-2-targeted antibody, in combination with chemotherapy regimens with or without pembrolizumab (an inhibitor of programmed cell death protein 1 (PD-1)) [6, 7]. Patients with HER-2 overexpression negative tumors but with programmed death ligand 1 (PD-L1) combined positive score (CPS) of 5 or above are recommended to receive nivolumab (another PD-1 inhibitor) in combination with chemotherapy [7]. For patients with adequate performance status who experience disease progression on first-line treatments, certain chemotherapy regimens have been shown to improve survival in the second and later lines of treatment when compared to best supportive care [6, 7]. Targeted therapies, such as ramucirumab (an inhibitor of vascular endothelial growth factor (VEGF)), are also recommended for these patients [7]. Despite this, around half of these patients will die within a year [8,9,10,11,12,13,14,15,16,17,18], indicating poor prognosis with the available treatments.
The comparative efficacy of interventions in the second and third lines of treatment for patients with advanced gastric cancer has previously been summarized [19,20,21,22]. The objective of this study was to synthesize the clinical prognosis of all-comer patients with pretreated advanced gastric cancer receiving relevant chemo- and targeted therapies (from here on referred to as the target population) through a meta-analysis of relevant randomized controlled trials (RCTs).
Methods
Study Identification
A comprehensive systematic literature review was conducted on July 6, 2021, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [23]. Two reviewers independently performed title/abstract screening, full-text screening, and data extraction of the included studies. At each stage, any discrepancies between reviewers were reconciled through discussion, with a third reviewer being involved to reach consensus on the remaining disagreements. The quality of the trials included in the meta-analysis was evaluated using the Cochrane Collaboration risk of bias assessment tool for RCTs [24]. Of note, the review protocol of this study was not registered with PROSPERO. We searched Embase, MEDLINE, CENTRAL, recent (2019–2021) conference proceedings, and the US clinical trials registry to identify clinical trials conducted in the target population and published since 2000. Search strategies for the systematic review are provided in Online Resource 1.
Population of interest was all-comer patients (i.e., regardless of specific biomarkers) with disease progression on or after at least one prior line of systemic therapy for advanced (unresectable and/or metastatic) gastric cancer. Interventions of interest were systemic therapies that were granted regulatory approval for any indication. Detailed study eligibility criteria of the systematic literature review are presented in Table 1.
Meta-Analysis
Overview
A meta-analysis was conducted including studies involving chemotherapies and/or targeted therapies relevant in treatment guidelines and HTA activities, as these classes of therapies are standard in treating the target population. Relevant therapies were as follows: folinic acid + 5-FU + oxaliplatin (FOLFOX), folinic acid + 5-FU + irinotecan (FOLFIRI), ramucirumab + paclitaxel, docetaxel, paclitaxel, carboplatin + paclitaxel, ramucirumab + docetaxel, capecitabine, irinotecan, trifluridine + tipiracil, ramucirumab, carboplatin + irinotecan, folinic acid + 5-FU + irinotecan + oxaliplatin (FOLFIRINOX), epirubicin + cisplatin + capecitabine (ECX), epirubicin + oxaliplatin + capecitabine (EOX), gemcitabine, 5-FU + carboplatin + docetaxel, 5-FU, cisplatin, docetaxel, 5-FU, and apatinib.
The primary outcome of interest was the objective response rate (ORR). Secondary outcomes were overall survival (OS) and progression-free survival (PFS) presented as Kaplan–Meier data. Due to the vast amount of literature identified in the systematic review, the study designs of interest for the analysis were limited to RCTs, as this type of trial design provides the highest quality of experimental evidence [25]. RCTs evaluating at least one of the outcomes of interest were eligible for inclusion, and data from all their treatment arms were incorporated into the analysis. Detailed study eligibility criteria of the meta-analysis are presented in Table 1.
Meta-analyses were performed to combine the results from multiple studies in an effort to obtain a precise estimate of the overall rate and/or to resolve uncertainty around the efficacy of treatments for target patients.
Due to inherent differences among the included trials, targeted treatments, dose intensities, study design, length of follow-up, populations, and outcome measurements, heterogeneity was expected. Therefore, a random-effects meta-analysis was used to synthesize the overall estimate of interest, although a fixed-effect model was also planned for in the case of a low number of included treatment arms. Post hoc independent subgroup analyses by therapy class were conducted in the case of high heterogeneity. To assess for potential publication bias, visual inspection of a funnel plot and Egger’s test were performed. Additional details on the statistical methods can be found in Online Resource 1.
Objective Response Rate
ORR is defined as the proportion of patients who achieved either complete response or partial response following treatment (i.e., number of responders/total participants). When performing meta-analyses of proportions, it is usually advantageous to first transform the proportions into a measure that has better statistical properties, particularly when the number of events is very small or zero. The Freeman-Tukey double arcsine transformation [26] was used to transform raw response proportion estimates so that the data follow an approximate normal distribution. This approach is recommended as it does not require any adjustments or continuity corrections to the observed data. The model estimate was then back-transformed such that the final estimate was on the original scale (proportion) [27]. Clopper-Pearson 95% confidence intervals (CIs) were computed for individual treatment arms.
Time-To-Event Survival Outcomes
Overall survival was defined as the time from the date of first dose to death by any cause. Progression-free survival was defined as the time from the date of first dose to disease progression or death by any cause, whichever occurs first. When summarizing OS and PFS, individual patient-level data were reconstructed from published Kaplan–Meier curves, using the Guyot algorithm [28] and DigitizeIt software version 2.3.3 (www.digitizeit.xyz), and pooling of these curves was performed. This approach enabled patient-level data from studies that only reported aggregated study-level data to be incorporated, allowing the use of time-dependent survival models in the meta-analysis. For the pooling of the reconstructed data across grouped studies, the conditional survival probabilities at each timepoint were arcsine transformed and modeled with a random effect meta-analysis method described by Combescure et al. [29] with study as the random term. The summary survival probabilities were obtained by the product of the pooled conditional survival probabilities. The mean and median survival times were derived from the summary survival curve assuming a linear interpolation of the survival between the points.
Software
SAS software version 9.4 [30] was used for conducting the meta-analyses of proportion outcomes R version 4.0.1 [31] was used for the generation of supporting forest plots. Pooling of survival curves was estimated using the metasurv package with R version 4.0.1. [31].
Presentation of Results
Results presented from the meta-analyses of ORR include fixed-effect and random-effects (pooled) estimates of ORR (with 95% CIs), \({I}^{2}\), \({\tau }^{2}\) statistics, p-value for Cochran’s Q test for testing heterogeneity, and corresponding forest plots. The \({I}^{2}\) statistic measures the percentage of variation across studies attributed to the heterogeneity among trials rather than chance. Results for each analyzed time-to-event endpoint include the total sample size, number of events, pooled survival curve, overall median survival time with the corresponding 95% CI, \({I}^{2}\) statistic, and p-value for Cochran’s Q test for testing heterogeneity.
Results
Evidence Base
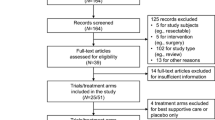
Searches from the main databases, conference proceedings, and other sources resulted in 14,148 records. After removing duplicate records (n = 4271) and excluding citations during title/abstract screening (n = 8908), 969 citations were advanced to the full-text screening stage. Of these, a total of 265 citations representing 214 unique clinical trials were included in the systematic literature review.
Of the 214 clinical trials included in the systematic literature review, 44 RCTs met the study eligibility criteria of the meta-analysis and were ultimately included (Fig. 1). Around half of the studies (n = 21 trials) were in phase II and the other half (n = 20) were in phase III; one was a phase II/III trial and two did not report the study phase. Twenty-six RCTs were open-label in design, with 15 being double-, triple-, or quadruple-blinded; three trials did not report on masking. Most of the studies (n = 40) were conducted in multiple centers. Thirty-five trials were published as full-text articles, five were published as conference abstracts, and four were only reported in the clinical trials registry. Ten trials were conducted globally and four were from Asia, with 11, seven, and five being exclusively conducted in Japan, Korea, and China, respectively; the remaining studies were conducted in Germany (n = 4), Japan and Korea (n = 1), the USA (n = 1), and the UK (n = 1). Trials often had low risk of bias in all evaluated dimensions and were of high quality (Fig. 2). Studies with “some concerns” regarding risk of bias were often only reported in conference abstracts or the clinical trials registry, with minimal information available on the randomization procedure.
Trial sample sizes ranged from 19 to 726 patients, with the proportion of males ranging from 57.1 to 81.9%. Mean age (and median age when mean was not reported) ranged from 52 to 68 years. Where reported, percentage of patients with Eastern Cooperative Oncology Group performance scores of either 0 or 1 ranged from 72.4 to 100%. Lastly, 36 trials were conducted in populations with exactly one prior line of treatment, whereas five trials were conducted in those with ≥ 2 prior lines; the remaining studies recruited patients with at least one prior line of treatment (Table 2).
Among treatment arms with active interventions, 38 unique regimens were evaluated in the included RCTs. Of these, eight were single-agent chemotherapies, 14 were combination chemotherapies, three were single-agent targeted therapies, nine were combinations of chemo- and targeted therapies, and two were chemotherapy agents in combination with other treatment classes. Lastly, two interventions (pembrolizumab and nimotuzumab + irinotecan) were immunotherapies and were therefore excluded from the meta-analysis.
Objective Response Rate
Seventy-seven treatment arms from 42 RCTs [9, 14,15,16,17,18, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] were included in the analysis. A funnel plot and Egger’s test were performed to assess potential publication bias in the literature (Fig. 3). Both visual inspection of the funnel plot and Egger’s test did not show any evidence of publication bias (intercept = 1.06, 95% CI−0.28 to 2.40, p = 0.12). The random effect ORR pooled estimate was 15.0% (95% CI 12.7–17.5%). The corresponding statistics for heterogeneity were \({I}^{2}\) = 85.3%, \({\tau }^{2}\) = 0.062, and \(p\) < 0.0001 (Fig. 4). Due to this high level of heterogeneity (I2 = 85.3%), a post hoc subgroup analysis by therapy class was conducted. Subgroups were defined by single-agent chemotherapies (n = 41 trial arms), combination chemotherapies (n = 12 trial arms), single-agent targeted therapies (n = 7 trial arms), and combination chemo- and targeted therapies (n = 17 trial arms). Meta-analyses revealed single-agent targeted therapies exhibited the lowest ORR at 8.6% (95% CI 3.0–16.3%, I2 = 89.8%), followed by single-agent chemotherapies at 14.0% (95% CI 11.6–16.7, I2 = 73.7%), combination chemotherapies at 15.5% (95% CI 9.5–22.6%, I2 = 79.3%), and combination chemo- and targeted therapies with the highest ORR at 20.9% (95% CI 15.7–26.7%, I2 = 87.4%).
Funnel plot for the assessment of publication bias. Funnel plot of the estimate in the random effects model of 77 trial arms included in the meta-analysis of objective response rate is symmetrical. Accompanying Egger’s test did not show any evidence of publication bias (intercept = 1.06, 95% CI −0.28 to 2.40, p = 0.12)
Overall Survival
Sixty-four treatment arms from 34 RCTs [9, 14,15,16,17,18, 32,33,34, 36, 38,39,40,41, 43,44,45,46,47, 52,53,54,55,56,57, 59,60,61, 62, 63, 66,67,68,69] were included in the analysis of overall survival. Median OS from the pooled analysis was 7.9 months (95% CI 7.4–8.5 months). Pooled OS rates at 6, 12, and 24 months were 62.5%, 30.4%, and 6.7%, respectively (Table 3). The corresponding statistics for heterogeneity were \({I}^{2}\) = 2.9% and \(p\) = 0.188 (Fig. 5A).
Kaplan–Meier estimates of overall survival and progression-free survival. The grey lines represent the Kaplan–Meier estimates for survival events in each treatment arm. The black squares represent the end of follow-up for each corresponding treatment arm. The thick black line represents the random effects pooled survival curve estimate with 95% confidence bands (dashed lines). P-value refers to Cochran’s Q test for heterogeneity. A Kaplan–Meier estimates of overall survival among 61 treatment arms included in the analysis. B Kaplan–Meier estimates of progression-free survival among 61 treatment arms included in the analysis
Progression-Free Survival
Sixty-one treatment arms from 32 RCTs [14,15,16,17,18, 32,33,34, 36,37,38,39,40,41, 43, 44, 46, 47, 52,53,54,55,56,57,58,59,60,61, 63, 66, 68, 69] were included in the analysis of progression-free survival. Median PFS from the pooled analysis was 3.5 months (95% CI 3.2–3.7 months). Pooled PFS rates at 6, 12, and 24 months were 25.8%, 7.3%, and 0.7%, respectively (Table 3). The corresponding statistics for heterogeneity were \({I}^{2}\) = 19.3% and \(p\) < 0.00001 (Fig. 5B).
Discussion
We aimed to synthesize and summarize all available relevant clinical evidence on the absolute efficacy of second- or later-line systemic therapies in patients with advanced gastric cancer to inform clinicians, patients, and healthcare decision makers. The target population included all those receiving a second or later line of treatment for advanced gastric cancer, regardless of the specific biomarkers that can guide the effective treatment [70], (i.e., HER-2 overexpression, high levels of microsatellite instability (MSI-H), mismatch repair deficiency (dMMR), programmed death ligand 1 (PD-L1) overexpression, and fibroblast growth factor receptor (FGFR) alterations). Outcomes of interest were ORR, OS, and PFS, as these were deemed the key endpoints in clinical trials of advanced gastric cancer.
Data from 7256 patients were incorporated in the analysis of ORR, with a total time-at-risk of 60,350, and 28,860 person-months for the analyses of OS and PFS, respectively. The estimated pooled ORR was 15.0%, and the median OS and PFS were 7.9 months and 3.5 months, respectively. To our knowledge, the current analysis is the only study that has quantitatively synthesized the clinical prognosis of patients treated with relevant chemo- and targeted therapies in the post-first-line setting. This is important because our findings indicate that clinical outcomes are still poor in the target population despite certain interventions being more efficacious than others, as shown in previously published analyses [20, 22, 71]. Furthermore, this research could be of value to many stakeholders including health technology assessment bodies, policy makers, physicians, patients, clinical development program scientists, and researchers. First, health technology assessment bodies and policy makers could use these results as a benchmark to contextualize the efficacy of novel treatments in the second- or later-line setting. Second, physicians could use these efficacy estimates in communicating with patients as well as in the medical decision-making process. Third, these results could be used in clinical development programs for defining hypotheses and statistical power calculations. Fourth, this research uses a state-of-the-art method of performing a meta-analysis on published, and then digitized survival curves. Researchers could further apply this technique to future applications in gastrointestinal cancer and other cancers.
Inference from a systematic review and meta-analysis approach has some limitations: the potential for publication bias should be noted although we performed hand searches of recent conference proceedings and clinical trial registry results to capture any available outcome data that may not have been published. In addition, a funnel plot and Egger’s test did not indicate any potential publication bias. Differences in observation periods could cause biased estimates for certain outcomes or treatments. Furthermore, although interventions recommended by clinical practice guidelines and relevant to health technology assessment submissions were included in the analyses, therapies used in routine clinical practice and/or approved in specific regions may not have been included. Immunotherapies were excluded from analyses as these therapies are currently only recommended in certain circumstances (e.g., microsatellite instability high (MSI-H), deficient mismatch repair (dMMR), high tumor mutation burden (TMB-high) tumors) and are not yet recommended for the broader, all-comers, pretreated population (NCCN Guidelines Version 2.2022). In addition, participants in the included studies are, for the vast majority, recorded before the introduction of these novel therapeutics. Lastly, there was heterogeneity among the studies in terms of phase of the trials, sample size, the region studies were conducted in, level of masking, and patient characteristics. Specifically, populations varied in terms of the number of prior lines of treatment, which may have affected the efficacy of the evaluated interventions. Since the results of individual trials depend on the trial design and participant characteristics, in the absence of individual patient-level data, potential underlying differences across the included trials (including the above-mentioned variables) could not be adjusted for beyond modelling with random effect meta-analysis. Note that the observed treatment effects across individual trials were less heterogeneous in terms of OS (\({I}^{2}\) = 2.9%) and PFS (\({I}^{2}\) = 19.3%) compared to ORR (\({I}^{2}\) = 85.3%).
Despite these limitations, the trials included in our analysis were identified based on a rigorous and comprehensive systematic review, which searched the published literature as well as recent conference proceedings and the US clinical trial registry based on pre-specified eligibility criteria. The entire systematic review was conducted by two reviewers, following PRISMA guidelines to ensure the accuracy and robustness of findings. For the analysis of survival outcomes, we not only used published OS and PFS rates, but also leveraged the information retrieved via digitizing the published Kaplan–Meier figures, thereby allowing the use of time-dependent survival models in the meta-analysis. The statistical approaches used in the meta-analysis were previously established in the published literature, and both fixed effect and random effects estimates were evaluated.
Overall, our findings are consistent with the current understanding of the efficacy of conventional chemotherapy and targeted therapies for pretreated patients with advanced gastric cancer, emphasizing that prognosis remains poor in those who receive them as salvage regimens [72]. Beyond these conventional treatments, however, various immunotherapies are approved, recommended, or being investigated in patients with advanced gastric cancer [7, 73]. Monoclonal antibodies against PD-1, its ligand (PD-L1), or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) can be administered as monotherapy or in combination with chemotherapy, targeted therapy (e.g., anti-HER-2 therapy, inhibitors of VEGF or its receptor (VEGFR)), or another immunotherapy [73, 74]. Specifically, patients with MSI-H/dMMR tumors and those with TMB-high are recommended to receive pembrolizumab, a PD-1 inhibitor, as second-line or subsequent therapy [7].
Our findings confirm poor prognosis among patients with advanced gastric cancer, with disease progression on first-line therapy. Payers, physicians, and patients could use these findings to contextualize the efficacy of novel therapies. Despite the approved, recommended, and experimental systemic treatments available, there is still an unmet need novel interventions for advanced gastric cancer patients receiving second or later lines of treatment.
Data Availability
The datasets analyzed during the current study were generated based on the data published in the citations included in this manuscript.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (London, England). 2019;393(10184):1948–57. https://doi.org/10.1016/s0140-6736(18)32557-1.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. https://doi.org/10.1056/NEJMoa055531.
Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol: Official J Am Soc Clin Oncol. 2011;29(13):1715–21. https://doi.org/10.1200/jco.2010.33.0597.
Society AC. Cancer Facts & Figures 2022. Atlanta: American Cancer Society; 2022.
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20.
Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022 Feb 1;20(2):167–92.
Cotes Sanchís A, Gallego J, Hernandez R, Arrazubi V, Custodio A, Cano JM, et al. Second-line treatment in advanced gastric cancer: data from the Spanish AGAMENON registry. PloS One. 2020;15(7):e0235848. https://doi.org/10.1371/journal.pone.0235848.
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78–86. https://doi.org/10.1016/s1470-2045(13)70549-7.
Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, Tsuji A, et al. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105(7):812–7. https://doi.org/10.1111/cas.12419.
Zhang DS, Jin Y, Luo HY, Wang ZQ, Qiu MZ, Wang FH, et al. Pemetrexed for previously treated patients with metastatic gastric cancer: a prospective phase II study. Br J Cancer. 2015;112(2):266–70. https://doi.org/10.1038/bjc.2014.607.
Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol : Official J Am Soc Clin Oncol. 2010;28(11):1904–10. https://doi.org/10.1200/jco.2009.26.2923.
Schønnemann KR, Yilmaz M, Bjerregaard JK, Nielsen KM, Pfeiffer P. Phase II study of biweekly cetuximab in combination with irinotecan as second-line treatment in patients with platinum-resistant gastro-oesophageal cancer. Eur J Cancer (Oxford, England : 1990). 2012;48(4):510–7. https://doi.org/10.1016/j.ejca.2011.12.005.
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438–44. https://doi.org/10.1200/JCO.2012.48.5805.
Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer. 2014;50(8):1437–45. https://doi.org/10.1016/j.ejca.2014.01.020.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9. https://doi.org/10.1016/S0140-6736%2813%2961719-5.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35. https://doi.org/10.1016/S1470-2045%2814%2970420-6.
Satoh T, Doi T, Ohtsu A, Tsuji A, Omuro Y, Mukaiyama A, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN - a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49. https://doi.org/10.1200/JCO.2013.53.6136.
Catenacci DV, Chao J, Muro K, Al-Batran SE, Klempner SJ, Wainberg ZA, et al. Toward a treatment sequencing strategy: a systematic review of treatment regimens in advanced gastric cancer/gastroesophageal junction adenocarcinoma. Oncologist. 2021;26(10):e1704–29. https://doi.org/10.1002/onco.13907.
Huang M, Li J, Yu X, Xu Q, Zhang X, Dai X, et al. Comparison of efficacy and safety of third-line treatments for advanced gastric cancer: a systematic review with Bayesian network meta-analysis. Front Oncol. 2021;11:734323. https://doi.org/10.3389/fonc.2021.734323.
Zheng Y, Zhu XQ, Ren XG. Third-line chemotherapy in advanced gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(24):e6884. https://doi.org/10.1097/MD.0000000000006884.
Zhu X, Ko YJ, Berry S, Shah K, Lee E, Chan K. A Bayesian network meta-analysis on second-line systemic therapy in advanced gastric cancer. Gastric Cancer. 2017;20(4):646–54. https://doi.org/10.1007/s10120-016-0656-7.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. https://doi.org/10.1016/j.jclinepi.2009.06.005.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. J Wiley & Sons, 2019.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–10. https://doi.org/10.1097/PRS.0b013e318219c171.
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950:607–11.
Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32(4):138.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. https://doi.org/10.1186/1471-2288-12-9.
Combescure C, Foucher Y, Jackson D. Meta-analysis of single-arm survival studies: a distribution-free approach for estimating summary survival curves with random effects. Stat Med. 2014;33(15):2521–37. https://doi.org/10.1002/sim.6111.
SAS Software version 9.4, Copyright © [2002–2012] SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. (2012). Accessed.
R: A Language and Environment for Statistical Computing. https://www.R-project.org/(2020). Accessed.
Bang YJ, Im SA, Lee KW, Cho JY, Song EK, Lee KH, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol. 2015;33(33):3858–65. https://doi.org/10.1200/JCO.2014.60.0320.
Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1637–51. https://doi.org/10.1016/S1470-2045%2817%2930682-4.
Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021;7(6):895–902. https://doi.org/10.1001/jamaoncol.2021.0275.
Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, Park Y, Koo DH, Lu JW, Xu J, Bhagia P, Kuang S, Shih CS, Qin S. Pembrolizumab vs paclitaxel as second-line treatment for Asian patients with PD-L1–positive advanced gastric or gastroesophageal cancer (GC) in the phase III KEYNOTE-063 trial. J Clin Oncol. 2020;38:15_suppl, e16586-e16586
Kang YK, Kang WK, Di Bartolomeo M, Chau I, Yoon HH, Cascinu S, Ryu MH, Kim JG, Lee KW, Oh SC, Takashima A. Randomized phase III ANGEL study of rivoceranib (apatinib)+ best supportive care (BSC) vs placebo+ BSC in patients with advanced/metastatic gastric cancer who failed≥ 2 prior chemotherapy regimens. Ann Oncol. 2019 Oct 1;30:v877-8.
Kang YK, Ryu MH, Park SH, Kim JG, Kim JW, Cho SH, et al. Efficacy and safety findings from DREAM: a phase III study of DHP107 (oral paclitaxel) versus i.v. paclitaxel in patients with advanced gastric cancer after failure of first-line chemotherapy. Ann Oncol. 2018;29(5):1220–6. https://doi.org/10.1093/annonc/mdy055.
Kim JY, Ryoo HM, Bae SH, Kang BW, Chae YS, Yoon S, et al. Multi-center randomized phase ii study of weekly docetaxel Versus weekly docetaxel-plus-oxaliplatin as a second-line chemotherapy for patients with advanced gastric cancer. Anticancer Res. 2015;35(6):3531–6.
Lee KW, Kim BJ, Kim MJ, Han HS, Kim JW, Park YI, et al. A multicenter randomized phase II study of docetaxel vs. docetaxel plus cisplatin vs. docetaxel plus S-1 as second-line chemotherapy in metastatic gastric cancer patients who had progressed after cisplatin plus either S-1 or capecitabine. Cancer Res Treat. 2017;49(3):706–16. https://doi.org/10.4143/crt.2016.216.
Lee KW, Maeng CH, Kim TY, Zang DY, Kim YH, Hwang IG, et al. A phase III study to compare the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer who failed in first-line therapy (KCSG ST10-01). Oncologist. 2019;24(1):18–24. https://doi.org/10.1634/theoncologist.2018-0142.
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-Arm, phase II trial. J Clin Oncol. 2013;31(26):3219–25. https://doi.org/10.1200/JCO.2013.48.8585.
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54. https://doi.org/10.1200/JCO.2015.63.5995.
Lorenzen S, Knorrenschild JR, Pauligk C, Hegewisch‐Becker S, Seraphin J, Thuss‐Patience P, Kopp HG, Dechow T, Vogel A, Luley KB, Pink D. Phase III randomized, double‐blind study of paclitaxel with and without everolimus in patients with advanced gastric or esophagogastric junction carcinoma who have progressed after therapy with a fluoropyrimidine/platinum‐containing regimen (RADPAC). Int J Cancer. 2020a Nov 1;147(9):2493–502.
Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, et al. Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin Oncol. 2020;38(17):1919–27. https://doi.org/10.1200/JCO.19.03077.
Maruta F, Ishizone S, Hiraguri M, Fujimori Y, Shimizu F, Kumeda S, et al. A clinical study of docetaxel with or without 5’DFUR as a second-line chemotherapy for advanced gastric cancer. Med Oncol. 2007;24(1):71–5. https://doi.org/10.1007/BF02685905.
Moehler M, Gepfner-Tuma I, Maderer A, Thuss-Patience PC, Ruessel J, Hegewisch-Becker S, et al. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: a randomized, placebo-controlled phase II AIO trial with serum biomarker program. BMC Cancer. 2016;16(1)(no pagination)(699). https://doi.org/10.1186/s12885-016-2736-9.
Nakanishi K, Kobayashi D, Mochizuki Y, Ishigure K, Ito S, Kojima H, et al. Phase II multi-institutional prospective randomized trial comparing S-1 plus paclitaxel with paclitaxel alone as second-line chemotherapy in S-1 pretreated gastric cancer (CCOG0701). Int J Clin Oncol. 2016;21(3):557–65. https://doi.org/10.1007/s10147-015-0919-z.
Irinotecan Hydrochloride With or Without Alvocidib in Treating Patients With Advanced Stomach or Gastroesophageal Junction Cancer That Cannot Be Removed By Surgery. ClinicalTrials.gov identifier: NCT00991952. https://ClinicalTrials.gov/show/NCT00991952
Assess the Efficacy of AZD8931 in Combination With Paclitaxel Versus Paclitaxel Alone in Patients With Gastric Cancer. ClinicalTrials.gov identifier: NCT01579578. https://clinicaltrials.gov/show/NCT01579578
A Study of BBI608 Plus Weekly Paclitaxel to Treat Gastric and Gastro-Esophageal Junction Cancer (BRIGHTER). ClinicalTrials.gov identifier: NCT02178956. https://clinicaltrials.gov/ct2/show/NCT02178956
A Study of Ramucirumab (LY3009806) in Combination With Paclitaxel in Participants With Gastric Cancer. ClinicalTrials.gov Identifier: NCT02514551. https://ClinicalTrials.gov/show/NCT02514551
Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M, et al. Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer. 2015;51(7):808–16. https://doi.org/10.1016/j.ejca.2015.02.009.
Roy AC, Park SR, Cunningham D, Kang YK, Chao Y, Chen LT, et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann Oncol. 2013;24(6):1567–73. https://doi.org/10.1093/annonc/mdt002.
Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer. 2015;18(4):824–32. https://doi.org/10.1007/s10120-014-0420-9.
Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48. https://doi.org/10.1016/S1470-2045%2818%2930739-3.
Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–87. https://doi.org/10.1016/S2468-1253%2816%2930219-9.
Shitara K, Yuki S, Tahahari D, Nakamura M, Kondo C, Tsuda T, et al. Randomised phase II study comparing dose-escalated weekly paclitaxel vs standard-dose weekly paclitaxel for patients with previously treated advanced gastric cancer. Br J Cancer. 2014;110(2):271–7. https://doi.org/10.1038/bjc.2013.726.
Su D. Clinical efficacy and safety of apatinib for treating stomach cancer and its effect on serum CA72-4, CEA and CA19-9. Acta Med Mediterr. 2020;36(3):1621–5.
Sym SJ, Hong J, Park J, Cho EK, Lee JH, Park YH, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol. 2013;71(2):481–8. https://doi.org/10.1007/s00280-012-2027-3.
Tanabe K, Fujii M, Nishikawa K, Kunisaki C, Tsuji A, Matsuhashi N, et al. Phase II/III study of second-line chemotherapy comparing irinotecan-alone with S-1 plus irinotecan in advanced gastric cancer refractory to first-line treatment with S-1 (JACCRO GC-05). Ann Oncol. 2015;26(9):1916–22. https://doi.org/10.1093/annonc/mdv265.
Lorenzen S, Thuss-Patience PC, Pauligk C, Goekkurt E, Ettrich TJ, Lordick F, Stahl M, Reichardt P, Soekler M, Pink D, Probst S. FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab as second-line therapy for patients with advanced or metastatic gastroesophageal adenocarcinoma with or without prior docetaxel: Results from the phase II RAMIRIS Study of the AIO. J Clin Oncol. 2020b May 20;38(15_suppl):4514–14. https://doi.org/10.1200/JCO.2020.38.15_suppl.4514.
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47(15):2306–14. https://doi.org/10.1016/j.ejca.2011.06.002.
Van Cutsem E, Bang YJ, Mansoor W, Petty RD, Chao Y, Cunningham D, et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol. 2017;28(6):1316–24. https://doi.org/10.1093/annonc/mdx107.
Wang F, Ren C, Xu J, Wang Z, He MM, Qiu M, Jin Y, Luo H, Wang F, Li Y, Tan Q. Apatinib plus paclitaxel versus placebo plus paclitaxel as second-line therapy in patients with gastric cancer with peritoneal carcinomatosis: A double-blind, randomized phase II trial. J Clin Oncol. 39, no. 15_suppl. https://doi.org/10.1200/JCO.2021.39.15_suppl.e16022.
Zhao X, Guo W, Chen Z, Zhang X, Zhu X, Zhang W, Yang L, Qiu L, Wang C, Huang M, Yu H, Li J, Zhang Z, Li W. Comparison of efficacy and safety of second-line palliative chemotherapy with paclitaxel plus raltitrexed and paclitaxel alone in patients with metastatic gastric adenocarcinoma: a randomized phase II trial. J Clin Oncol. 37, no. 15_suppl (May 20, 2019) 4054-4054. https://doi.org/10.1200/JCO.2019.37.15_suppl.4054.
Xu RH, Zhang Y, Pan H, Feng J, Zhang T, Liu T, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(12):1015–24. https://doi.org/10.1016/S2468-1253(21)00313-7.
Yi JH, Lee J, Park SH, Park JO, Yim DS, Park YS, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer. 2012;106(9):1469–74. https://doi.org/10.1038/bjc.2012.100.
Fushida S, Kinoshita J, Kaji M, Oyama K, Hirono Y, Tsukada T, et al. Paclitaxel plus valproic acid versus paclitaxel alone as second-or third-line therapy for advanced gastric cancer: a randomized phase II trial. Drug Des Dev Ther. 2016;10:2353–8. https://doi.org/10.2147/DDDT.S110425.
Nishina T, Boku N, Gotoh M, Shimada Y, Hamamoto Y, Yasui H, et al. Randomized phase II study of second-line chemotherapy with the best available 5-fluorouracil regimen versus weekly administration of paclitaxel in far advanced gastric cancer with severe peritoneal metastases refractory to 5-fluorouracil-containing regimens (JCOG0407). Gastric Cancer. 2016;19(3):902–10. https://doi.org/10.1007/s10120-015-0542-8.
Kahraman S, Yalcin S. Recent advances in systemic treatments for HER-2 positive advanced gastric cancer. Onco Targets Ther. 2021;14:4149–62. https://doi.org/10.2147/OTT.S315252.
Zheng T, Jin J, Zhang Y, Zhou L. Efficacy and safety of paclitaxel with or without targeted therapy as second-line therapy in advanced gastric cancer: a meta-analysis. Medicine (Baltimore). 2020;99(25):e20734. https://doi.org/10.1097/md.0000000000020734.
Smyth EC, Moehler M. Late-line treatment in metastatic gastric cancer: today and tomorrow. Ther Adv Med Oncol. 2019;11:1758835919867522. https://doi.org/10.1177/1758835919867522.
Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188615. https://doi.org/10.1016/j.bbcan.2021.188615.
Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30. https://doi.org/10.1056/NEJMoa2004413.
Funding
Financial support for the research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Lauren A. Abderhalden, Ping Wu, Mayur M. Amonkar, Andrew M. Frederickson, and Ali Mojebi. Analysis was performed by Lauren A. Abderhalden, Mayur M. Amonkar, Brian M. Lang, Sukrut Shah, and Fan Jin. The first draft of the manuscript was written by Ali Mojebi and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
Lauren A. Abderhalden, Brian M. Lang, Mayur M. Amonkar, Sukrut Shah, and Fan Jin are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and own stock in Merck & Co., Inc., Rahway, NJ, USA. Ping Wu, Andrew M. Frederickson, and Ali Mojebi are employees of PRECISIONheor, a healthcare research consultancy which received funding from Merck and Co., Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abderhalden, L.A., Wu, P., Amonkar, M.M. et al. Clinical Outcomes for Previously Treated Patients with Advanced Gastric or Gastroesophageal Junction Cancer: A Systematic Literature Review and Meta-Analysis. J Gastrointest Canc 54, 1031–1045 (2023). https://doi.org/10.1007/s12029-023-00932-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-023-00932-5