Abstract
Introduction
Studies investigating the effects of SARS-CoV-2 on male reproductive function are few and heterogeneous, and results are often conflicting. This systematic review and meta-analysis was carried out on studies conducted in men with active or anamnestic SARS-CoV-2 infection to evaluate its consequences on the male sex hormone profile and semen parameters.
Materials and method
This meta-analysis follows the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocols. PubMed, Scopus, Cochrane, and Embase databases were searched to identify relevant studies. We originally selected 3553 articles. After the eligibility phase, 16 articles met our inclusion criteria encompassing 11 case-control studies and 5 cohort studies (2 prospective and 3 retrospective studies). We performed the quantitative analysis with Comprehensive Meta-Analysis Software. Cochran-Q and heterogeneity (I2) indexes were used to assess statistical heterogeneity. Sensitivity analysis and publication bias tests were also performed.
Results
Overall, 1250 patients with active or recent (up to 80 days before) COVID-19 infection and 1232 matched healthy controls were included. Sperm concentration, total sperm count, and total motility were significantly lower in patients compared with controls. Patients also showed lower levels of total testosterone and follicle-stimulating hormone, and higher levels of luteinizing hormone, 17β-estradiol, and prolactin compared with healthy controls. None of the included studies found the presence of SARS-CoV-2 mRNA in the semen of infected patients.
Conclusion
The present systematic review and meta-analysis suggests the presence of an association between SARS-CoV-2 infection and primary testicular damage manifested with a picture of altered steroidogenesis and worsening spermatogenesis. The absence of the virus in the seminal fluid indicates a low possibility of sexual transmission of the infection to partners and offspring. However, our findings mostly show short-term follow-up, while few studies have considered the long-term consequences of the viral infection, thus further studies are needed to evaluate the long-term consequences on male reproductive health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) epidemic broke out in China, in the city of Wuhan, in December 2019 [1]. The virus belongs to the Coronaviridae family and probably originated by spill-over from animal species. However, it shares many sequences with SARS-CoV1, a virus of the same family that gave rise to the Middle-East Respiratory Syndrome epidemic, which spread out from Saudi Arabia to the Middle East in 2012. SARS-CoV-2 was quickly shown to be extremely virulent and pathogenic, causing fatal interstitial pneumonia in a significant proportion of cases. On December 31st, 2019, WHO declared a pandemic status, as the virus had spread to every country of the world and every government moved to put in place collective safety and security regimes to halt the progression of the virus. To date, SARS-CoV-2 has generated 670 million cases and 6 million deaths worldwide (www.worldometers.info; https://www.worldometers.info/coronavirus/).
The interest of scientific research has shifted toward identifying the factors predisposing to infection and the severity of the disease. Current evidence has demonstrated that the mortality rate from COVID-19 depends on comorbidities, increasing progressively in patients with cardiovascular disease (CVD), diabetes mellitus, or hypertension [2]. Another conditioning factor is represented by age since only a small part of deaths is represented by the population under 50 years of age, while the greatest number of deaths is identified in the age groups over 80 years. These statistics appear to be present worldwide [3].
Epidemiological data of SARS-CoV-2 after the outbreak demonstrated a higher prevalence and severity in men, demonstrating a greater susceptibility to infection, a greater likelihood of a more aggressive course of the disease, and a greater prevalence of mortality [4, 5]. Despite a likely multi-factorial etiology, these earlier data may partially be explained by the expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in the male genital tract [6]. However, there is little evidence in vivo of orchitis linked to COVID-19. Additionally, few studies identified the SARS-CoV-2 in the semen and the possibility of sexual transmission is still a matter of concern [7]. This does not, however, rule out the possibility of unintended consequences on testicular function [8]. Though indirect damage brought on by inflammation, medications, and fever appears to be temporary, the virus does not appear to directly harm testicular function. However, fever has been shown to have negative effects on sperm quality [9].
To confirm these hypotheses, epidemiological data report that patients with hypogonadism seem to develop more complications [10]. Different suggestions have been proposed to explain these mechanisms. The first is based on the different hormonal milieu between the two sexes and implies a modulatory role of estrogens in the inflammatory state, which gives greater protection to women. However, testosterone also maintains an anti-inflammatory effect by reducing the cytokines most involved in systemic inflammation (IL-1, IL-6, TNFα). Indeed, several studies [11,12,13,14,15,16] have identified a worse prognosis in patients with known hypotestosteronemia, with a prolonged disease course, and longer stay in the Intensive Care Unit.
The virus is capable of infecting Leydig cells and it has been postulated that infected patients may develop hypogonadism [17]. Hypogonadism, and thus decreased total testosterone (TT) levels, can lead to destabilization of the immune response and endothelial dysfunction, resulting in impaired virus clearance and massive inflammatory response [18].
However, it is not yet known whether testosterone deficiency is a cause or a consequence of COVID-19 and whether it results from primary gonadal damage or hypothalamic-pituitary-testicular axis dysfunction [19]. It is readily apparent that COVID-19 can disrupt the hypothalamic-pituitary-gonadal (HPG) axis [20, 21]. The pathological state of steroidogenesis in the testis was reflected by variations in T levels, which were connected to the dysregulated levels of LH and FSH in COVID-19-affected individuals. In addition, the decreased T levels may result in altered spermatogenesis and erectile dysfunction, which support subfertility [22]. Higher levels of LH and FSH are indicative of adverse outcomes such as testicular injury [23]. In addition to hypothyroidism, dysregulation of the HPG axis has been linked to chronic renal illness, liver cirrhosis, and neurodegenerative senescence [24]. It must be said pharmacological interference cannot be excluded, since the mainstay of anti-inflammatory therapy is based on the administration of high doses of glucocorticoids which may affect the hypothalamus, resulting in suppression of pulsatile gonadotropin-releasing hormone (GnRH) secretion leading to central hypogonadism [25, 26].
Furthermore, the different hormonal milieu appears to play a role in the modulation of ACE2 expression [27]. ACE2 is part of the renin-angiotensin system; it is mainly located in the alveolar epithelium, although recent studies have identified a higher concentration in Leydig and Sertoli cells. SARS-CoV-2 has a high affinity for ACE2 which constitutes the entry gate of the virus into cells [6, 28, 29]. In addition, ACE2, as well as TMPRSS2, another protein implicated in the mechanism of virus entry into cells, has been found in spermatogonia and spermatids. Thus, a picture of viral testicular colonization is configured, probably capable of altering spermatogenesis [6, 30,31,32,33,34,35,36].
Also of particular concern is the viral localization in semen and the possible risk of its sexual transmission. Indeed, the virus has been isolated in the semen of infected patients. A review by Abdollahpour et al. confirmed sexual transmission of the virus in 16 out of 21 studies [37]. These implications acquire greater importance not only for the possible source of infection and extent of virus containment measures but also for the possible embryo and gestational damage that may result from spermatogonial infection. Indeed, the role of the virus in spermatogonia has yet to be investigated in scientific studies, as well as any consequences on conception and embryo health [38].
Numerous meta-analyses on the short-term impact of SARS-CoV-2 infection on testicular function have already been published. However, most studies only reported the effects on LH, FSH, and TT serum levels. Understanding the impact of the infection also on serum PRL, E2, and SHBG levels would be useful to have a more complete picture of the pathogenetic mechanisms through which the infection causes testicular dysfunction. Furthermore, not all studies included in previous meta-analyses consider positivity to SARS-CoV-2 on RT-PCR analysis of nasal or pharyngeal swabs and/or positive serology for IgA/IgA antibodies as the main criteria of COVID-19 diagnosis. Some studies, for example, only considered radiological images, making the diagnosis of SARS-CoV-2 infection uncertain.
With these premises, this systematic review and meta-analysis aims to comprehensively evaluate the effects of active or anamnestic SARS-CoV-2 infection on testicular function and, in particular, on the hypothalamic-pituitary-testicular axis and sperm parameters. Specifically, we wanted to analyze a wide range of hormones in patients in whom the diagnosis of COVID-19 was based on positivity to SARS-CoV-2 on RT-PCR analysis of nasal or pharyngeal swabs and/or positivity in serology for IgA/IgA antibodies.
Material and methods
Search strategy
This systematic review and meta-analysis was carried out according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Protocols. The literature search was performed up to December 15, 2022. An extensive search of the PubMed, Scopus, Cochrane, and Embase databases was performed, focusing on the effects of SARS-CoV-2 on male and female reproductive function, including testicular function, ovarian function, sex hormones profile, and pregnancy outcomes. To accomplish this, the following key strings were used: TITLE-ABS-KEY (“COVID19” OR “COVID-19” OR “SARS-CoV-2” OR “COVID” OR “SARS-CoV” OR “coronavirus” OR “SARS” OR “SARS-CoV”) AND TITLE-ABS-KEY (“Sertoli” OR “Leydig” OR “testicle” OR “testis” OR “spermatozoa” OR “sperm” OR “spermatogenesis” OR “fertility” OR “testosterone” OR “FSH” OR “LH” OR “hypogonadism” OR “ovary” OR “estradiol” OR “ovulation” OR “granulosa” OR “oocyte” OR “pregnancy” OR “ART” OR “assisted reproductive tech*” OR “IVF” OR “in vitro fertil*” OR “ICSI” OR “intracytoplasmic sperm injection” OR “IUI” OR “intrauterine insemination” OR “miscarriage” OR “LBR” OR “live birth rate”) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (LANGUAGE, “English”)). Additional manual searches were conducted using the reference lists of the relevant studies. The search was limited to English language studies.
Selection criteria
Articles were assessed for eligibility using the Population, Exposure, Comparison/Comparator, Outcome, and Study type (PECOS) model system [39]. Specifically, we included articles on men with active or anamnestic SARS-CoV-2 infection comparing their testicular function (conventional sperm parameters and sex hormones) with that of uninfected men. Unhealthy men suffering from urogenital infections, which would have influenced the results, were excluded. Articles documenting the presence of SARS-CoV-2 RNA in the seminal fluid of infected patients were also included. Original human studies were included, while animal studies, case reports, and non-original studies such as reviews or comments were excluded from the analysis (Table 1).
Articles selection was performed independently by two authors (M.M. and R.C). The titles and abstracts of the studies were first screened independently for inclusion. The decisions were reviewed by two reviewers unblinded (R.A.C. and S.L.V.). Disagreements between the authors were resolved by reviewers, while disagreements between the reviewers were resolved by a senior author (A.E.C.). Finally, the eligible articles underwent data extraction.
Data extraction
Information was collected on the first author, year of publication, study design, duration of infection (if available), age, body mass index (BMI), LH, follicle-stimulating hormone (FSH), TT, prolactin, 17β-estradiol (E2), sex hormone-binding globulin (SHBG), sperm concentration, total sperm count, progressive and total motility, SARS-CoV-2 mRNA in semen in cases and controls. These parameters were collected even if reported stratified according to the severity of the COVID-19 infection. When a value was available in a different unit of measure, it was converted according to the conversion tables. For each parameter, the number of people (COVID positive/COVID negative), mean value, standard deviation (SD), median value, and interquartile range (IQR) range were reported. For the studies expressing data as median and IQR, the formula by Wan et al. [40] was used to estimate the mean and SD. Data were independently extracted by two authors. Differences between reviewers were discussed until a consensus was reached.
Quality assessment
The quality of evidence (QoE) of the included studies was assessed by two researchers (M.M. and R.C.) using the Cambridge Quality Checklists [41]. Any disagreement between the two investigators was resolved through discussion with the other two researchers (R.A.C. and S.L.V.).
The Cambridge Quality Checklists [41] consists of three checklists to identify high-quality studies of (1) correlates, (2) risk factors, and (3) causal risk factors. The correlates checklist evaluates the appropriateness of the sample size and the quality of the outcome measurements. The risk factor checklist assigns high-quality scores to those studies with appropriate time-ordered data. Finally, the causal risk factors checklist assesses the type of study design. To draw confident conclusions about correlates, risk factors, and causal risk factors, all three checklist scores must score high.
Statistical analysis
Quantitative data analysis was performed using Comprehensive Meta-Analysis Software (Version 3) (Biostat Inc., Englewood, NJ, USA). Standardized mean difference (SMD) has been calculated for statistical comparison between cases and controls. Statistical significance was accepted for p values less than or equal to 0.05. The Cochran-Q and heterogeneity indexes (I2) were used to assess statistical heterogeneity. In particular, if I2 was less than or equal to 50%, the variation of the studies was considered homogenous and the fixed effect model was adopted to calculate the pooled effect size. However, if I2 was greater than 50%, there was significant heterogeneity between studies, and the random effects model was used. Publication bias was qualitatively analyzed by the asymmetry of the funnel plot, which suggested some missing studies on one side of the graph. For quantitative analysis of publication bias, we used Egger’s intercept test, which assessed the statistical significance of publication bias. In this occurrence, unbiased estimates were calculated using the “trim and fill” method.
Results
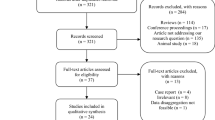
Using the aforementioned search strategy, we found 3553 articles that were screened by title and abstract. Of these, 3237 were judged not pertinent to reading their abstracts, for the following reasons: 2894 reported a different topic, 259 did not report information about SARS-Cov-2 infection, and 84 were excluded as they were reviews, editorials, case reports, or book chapters. The remaining 316 articles were evaluated for eligibility based on the reading of their full text. Of these, 300 were excluded, with reasons (Fig. 1). The remaining, 16 articles met our inclusion criteria. They included a total of 1250 patients with an active or anamnestic COVID-19 infection and 1232 age- and BMI-matched healthy controls with no prior diagnosis of urological or andrological disease. The articles included in our study were divided into two different groups: a group of 7 articles [42,43,44,45,46,47,48,49] evaluated the effect of SARS-CoV-2 infection on sperm parameters in cases and controls and a group of 12 cohort articles [20, 21, 23, 44, 49,50,51,52,53,54,55,56] evaluated the impact of SARS-CoV-2 on one or more serum hormones (LH, FSH, TT, prolactin, E2, and SHBG). Of these, 7 assessed LH and FSH [20, 21, 23, 44, 49,50,51, 55], 8 assessed TT [20, 23, 42, 49,50,51, 53,54,55, 57], 4 assessed E2 levels [42, 50, 54, 55], 3 analyzed prolactin levels [42, 44, 50], and 2 reported the values of SHBG [53, 54]. The main characteristic of the studies selected for meta-analysis is described in Table 2.
Quality of evidence of included studies
The quality of evidence (QoE) of the studies was assessed by 2 investigators (RC and MM), using the Cambridge Quality Checklist [41]. Although this scale does not establish a precise threshold for differentiating between high and low-quality studies, out of a total score of 15, 1 study scored >10, the remaining studies scored 6 to 10. No study achieved a score lower than 6 (Table 3).
Age in patients vs. controls
Pooled analysis of the 16 studies revealed that age was not significantly different between patients and controls (SMD 0.211; CI 95% −0.205, 0.628; p = 0.320) (Supplementary Fig. 1). The analysis showed the presence of heterogeneity between studies as shown by the I2 test (I2 = 91%, p = 0.000) and the Q-test (Q-value = 102.594). We found the absence of publication bias, as shown by the symmetry of the funnel plots (Supplementary Fig. 2A), and the result of Egger’s test (intercept −2.39430, 95% CI −7.27079, 2.48249; p = 0.14517). Any study was found sensitive enough to alter the results (Supplementary Fig. 2B).
BMI in patients vs. controls
Seven studies [20, 48,49,50, 53, 58, 59] reported information on BMI for patients and controls. BMI was not significantly different (SMD 0.341; CI 95% −0.043, 0.724; p = 0.082) (Supplementary Fig. 3). The analysis showed heterogeneity between studies (I2 = 87%, p = 0.000; Q = 55.314) and absence of publication bias, as derived from the symmetry of the funnel plots (Supplementary Fig. 4A) and Egger’s test result (intercept −0.59774, 95% CI −5.71742, 4.52194; p = 0.39236). Two studies were sensitive enough to alter the results [20, 50] (Supplementary Fig. 4B).
LH in patients vs. controls
Seven studies [20, 23, 42, 49, 51, 53, 55] including 824 patients assessed serum LH levels. Our analysis showed that LH levels were significantly higher in patients than in controls (SMD 1.229; CI 95% 0.372, 2.087; p = 0.005) (Fig. 2). The analysis showed the presence of heterogeneity between studies, as derived by the I2 test (I2 = 97%, p = 0.000), and the Q-test (Q = 278.960). The analysis showed the absence of publication bias as inferred by Egger’s test (intercept 6.91812, 95% CI −4.80247, 18.63892; p = 0.094) and the symmetry of the funnel plots (Supplementary Fig. 5A). No study was sensitive enough to alter the results (Supplementary Fig. 5B).
FSH in patients vs. controls
Seven studies [20, 23, 42, 49,50,51, 58], with a total of 808, reported serum FSH levels. Pooled analysis showed that FSH levels were significantly lower in patients vs. controls (SMD −0.934, CI 95% −1.647, −0.222, p = 0.010) (Fig. 3). The analysis showed the presence of heterogeneity between studies as shown by the I2 test (I2 = 97%, p = 0.000) and the Q-test (Q = 209.335). The analysis showed the absence of publication bias, as shown by the funnel plots and Egger’s test results (intercept 6.477846, 95% CI −16.30546, 3.34855; p = 0.07546) (Supplementary Fig. 6A). One study was sensitive enough to alter the results [49] (Supplementary Fig. 6B).
Total testosterone in patients vs. controls
We conducted an overall analysis of serum TT levels expressed in nmol/l. Eight studies [20, 42, 49,50,51, 53, 54, 58] reported TT levels in a total of 887 patients. Our analysis showed that TT levels were significantly lower in patients than in controls (SMD −0.983, CI 95% −1.769, −0.0197, p = 0.014) (Fig. 4). I2 and Q tests showed the presence of heterogeneity between studies (I2 = 97.8%, p = 0.000; Q = 317.934). The analysis showed the absence of publication bias, as shown by the symmetry for the funnel plots (Supplementary Fig. 7A) and Egger’s test (intercept 2.23248, 95% CI −14.00580, 18.46877; p = 0.37406). One study was sensitive enough to alter the results [49] (Supplementary Fig. 7B).
Prolactin in patients vs. controls
We conducted our analysis on three studies that reported serum prolactin levels in a total of 90 patients [44, 50, 52]. We found that the levels of this hormone were significantly higher in patients than in healthy controls (SMD 0.385, CI 95% 0.077, 0.693, p = 0.014) (Fig. 5). The analysis showed no heterogeneity between the studies (I2 = 12.6%, p = 0.32; Q-test = 2.289). No publication bias was revealed in the analysis, as shown by the symmetry of the funnel plots (Supplementary Fig. 8A) and Egger’s test (intercept −3.12696, 95% CI −12.92849, 6.67458; p = 0.07699). The sensitivity analysis revealed one study [42] that could alter the results (Supplementary Fig. 8B).
17ß-estradiol in patients vs. controls
We found four studies [42, 50, 54, 58] that evaluated serum E2 levels in a total of 390 patients. The values were reported in pg/ml. Our analysis found that E2 levels were significantly higher in patients than in healthy controls (SMD 0.524, CI 95% 0.113, 0.935, p = 0.012) (Fig. 6). The I2 test showed heterogeneity between studies (I2 = 76.8%, p = 0.005; Q = 12.932). The analysis showed the absence of publication bias, as shown by the symmetry of the funnel plot (Supplementary Fig. 9A) and the results of Egger’s test (intercept –2.67325, 95% CI −10.26546, 4.91896; p = 0.13450). Two studies were sensitive enough to alter the results [50, 55] (Supplementary Fig. 9B). In the study of Xu et al. [50], as explained by the authors, this could be related to the E2 measurement technique, which was done by radioimmunoassays. The authors suggest that it has lower precision and sensitivity than the new analysis techniques and may cause measurement errors.
SHBG in patients vs. controls
We evaluated two studies [53, 54] that reported SHBG values in 79 COVID-19 patients. The analysis showed that serum SHBG levels were not significantly different in patients compared to controls (SMD 0.088, CI 95% −0.243, 0.419, p = 0.603) (Fig. 7). The analysis showed no heterogeneity between the studies (I2 = 0%, p = 0.47, Q = 0.526). Analysis of publication bias could not be performed due to the limited number of studies. Neither study was found sensitive enough to alter the results (Supplementary Fig. 10).
Sperm concentration in patients vs. controls
Five studies [42, 44, 46, 48, 49] involving 207 patients evaluated sperm concentration. Our analysis revealed that sperm concentration was significantly lower in patients than in healthy controls (SMD −0.383, CI 95% −0.58, −0.186, p = 0.000) (Fig. 8). The analysis showed no heterogeneity between the studies (I2 = 40%, p = 0.13, Q = 8.373). Also, the symmetry of the funnel plots (Supplementary Fig. 11A) and the results of Egger’s test (intercept 0.57986, 95% CI −3.38352, 4.54323; p = 0.35269) showed the absence of publication bias. No study was sensitive enough to alter the results (Supplementary Fig. 11B).
Total sperm count in patients vs. controls
Five studies [42, 44, 46, 48, 49] involving 214 patients evaluated total sperm count. The analysis showed significantly lower total sperm count in patients than in healthy controls (SMD −0.682, CI 95% −0.889, −0.475, p = 0.000) (Fig. 9). Heterogeneity was found between studies (I2 = 91%, p = 0.000, Q = 58.291). The analysis showed the absence of publication bias, as shown by the symmetry of the funnel plot (Supplementary Fig. 12A) and Egger’s test (intercept −0.63931, 95% CI −11.27831, 9.99969; p = 0.43780). No study was sensitive enough to alter the results (Supplementary Fig. 12B).
Progressive motility in patients vs. controls
Two studies [42, 46] evaluated progressive sperm motility, which was not significantly different in the 96 patients enrolled in both studies compared to healthy controls (SMD −0.083, CI 95% −0.964, 0.799, p = 0.854) (Fig. 10). I2 and the Q tests showed the presence of heterogeneity between studies (I2 = 91%, p = 0.001, Q = 11.335). Analysis of publication bias could not be performed due to the limited number of studies. Sensitivity analysis revealed that both studies were sensitive enough to alter the results (Supplementary Fig. 13). Interestingly, Guo et al. [42], in the subgroup analysis of men who provided a second semen sample, obtained 80 days (IQR 74.0–89.0) after hospital discharge, found no significant improvement in progressive motility.
Total motility in patients vs. controls
Total sperm motility was evaluated in two studies [42, 46] involving 96 patients. Our analysis showed that total motility was significantly lower in patients than in controls (SMD −0.589, CI 95% −0.842, −0.337, p = 0.000) (Fig. 11). The analysis showed no heterogeneity between studies (I2 = 0%, p = 0.817, Q = 0.053). Analysis of publication bias could not be performed due to the limited number of studies. Sensitivity analysis found that these studies were not sensitive enough to alter the analysis (Supplementary Fig. 14).
SARS-CoV-2 mRNA detection
Seven studies [44,45,46,47,48,49, 60] evaluated the presence of SARS-CoV-2 mRNA in the semen of patients and controls. None of them found viral mRNA in the semen.
Discussion
Background literature
Since the COVID-19 outbreak, many studies have been conducted to evaluate the consequences of acute SARS-CoV-2 infection. These mainly concern respiratory function and the disease-related severe inflammation, resulting in an acute multidistrict inflammatory reaction, but many human apparatuses and systems are also affected by the virus. Thus, research interest has mostly focused on the prevention and management of life-threatening conditions, while little is known about the effects of COVID-19 on the endocrine and genital systems. While long-term sequelae of virus exposure are not yet clearly detectable, an increased prevalence of orchitis and epididymitis has been reported in the pandemic years, suggesting the involvement of the virus in acute male urogenital tract infection [8, 61]. This has been the subject of cohort studies, case reports, and trials [8, 47, 61,62,63,64]. Retrospective and prospective studies have also been conducted to evaluate the influence of the virus on sperm parameters and male sex hormones [47, 64].
In this regard, we reviewed data from four recently published meta-analyses. Of these, two investigated the effects of the virus both on sex steroid hormones and sperm parameters [65, 66], while the other two only reported data from studies on semen analysis [67, 68].
Specifically, the studies reported LH, FSH, TT, semen volume, sperm concentration, total sperm count, total, non-progressive, and progressive sperm motility, and sperm morphology. Only one meta-analysis [65] included studies reporting E2 and prolactin levels, while none of them considered SHBG values. The results of these meta-analyses are summarized in Table 4.
The finding of low serum TT in patients with current or previous COVID-19 infection achieved statistical significance in both meta-analyses evaluating the effect of the virus on sex hormones [65, 66]. These data are in agreement with the results of our study. In contrast to our data, which reported increased LH levels in infected patients, a non-significant difference in LH and FSH levels was reported in both meta-analyses. The only meta-analysis that investigated E2 and prolactin levels reported a statistically significant increase in both hormones in patients compared with controls [65], consistent with our results. A significant reduction in semen volume was reported in two meta-analyses [66, 67], while the other two did not report a statistically significant difference. Sperm concentration, and total sperm count were significantly reduced in infected patients in the meta-analyses by Corona et al. [66], Xie et al. [67], and Wang et al. [65], while Chen et al. [68] found no significant differences between the two groups. Progressive sperm motility has been reported to be reduced in patients compared to controls by Xie et al. [67] and Wang et al. [65], but not by Corona et al. [66] and Chen et al. [68]. These results are in line with ours, except for progressive motility for which we did not find a statistically significant difference. Finally, only one meta-analysis [65] evaluated the effects of infection on non-progressive motility which was not statistically different between patients and controls. Total motility was significantly affected in all analyses, as also reported in the present study. Finally, the studies evaluating sperm morphology showed no statistically significant effect on this parameter by SARS-CoV-2 infection. Concerning the follow-up time, these meta-analyses mainly included patients with active or recent infection, while it is unknown whether these results would be confirmed after longer follow-up times. Corona et al. [66] conducted their study on hormonal profile and semen samples collected from patients in the acute phase of the disease. Xie et al. [67] guided the study on semen samples from an average of 14–40 days after positivity for the virus. Wang et al. [65] data on the hormonal study were performed >90 days from positivity, therefore in a recovery phase. The authors themselves conclude that a longer follow-up would be necessary to evaluate the consequences of COVID on male hormone profile. In the same study, semen samples were instead collected at a median time ≤90 days after infection, in the acute phase of viral infection, to assess the consequences of the virus on male gametes. Similarly, Chen et al. [68] conducted their study on semen sampled 90 days after infection.
Our findings
In line with previous evidence, we analyzed the data of a short follow-up time in keeping with most of the data published so far. Indeed, hormonal evaluation and sperm analysis were performed in a period ranging from baseline (acute infection) up to 80 days from infection. We found a decrease in TT levels in male patients with active or anamnestic COVID-19 infection, thus a condition of hypotestosteronemia that could alter the response of male patients to the infection and compromise spermatogenesis. This alteration suggests the direct role of COVID-19 on testicular function, also supported by the finding of an alteration of spermatogenesis. The mechanisms that cause hypotestosteronemia may relate to inflammation. Indeed, SARS-CoV-2 infection causes inflammation and increases oxidative stress [69, 70], which leads to reduced androgen synthesis and, in parallel, generates increased E2 biosynthesis due to increased aromatase activity [71]. The increased activity of this enzyme could be due to obesity. The latter is a known risk factor for severe COVID-19 [72] and shares with this disease some common mechanisms, such as immune system activity attenuation and chronic inflammation [73]. Furthermore, obesity, and, in particular visceral obesity, is a well-known risk factor for testosterone deficiency in men [74,75,76]. However, we found no differences in BMI between patients and controls. This observation makes a role of obesity in the pathogenesis of hypotestosteronemia unlikely, while it suggests a role of SARS-CoV2 infection.
Our analysis confirms other data in the literature showing that low testosterone levels are associated with high LH levels, while FSH secretion does not appear to change significantly. Some studies, including our meta-analysis, have also shown that prolactin is significantly higher in patients with COVID-19 than in healthy men [77]. This could be due to the stress of being unwell and/or the medications being administered, but the exact mechanism and long-term consequences of elevated prolactin levels are still unclear. Hyperprolactinemia has been suggested to play a role in the pathophysiology of the hypogonadism observed in male patients with COVID-19 [78]. Furthermore, Guo et al. [42] suggested that hyperprolactinemia may be pre-existing, as no recovery was observed in a second blood draw after viral clearance; this could then alter the sensitivity analysis. Recovery time from hyperprolactinemia could provide insight into the time to the improvement of male gonadal function. Therefore, hormonal surveillance studies are needed to evaluate the influence on gonadal function and recovery time. Undoubtedly, we question a possible pathogenetic role of hyperprolactinemia in the onset of hypogonadism in patients with COVID-19 because these patients also have significantly elevated LH levels, whereas hyperprolactinemia is known to be associated with low or inappropriately low LH levels. Therefore, according to the current evidence, hypogonadism in COVID-19 patients mainly seems to be recognized as a primary mechanism. Analysis of SHBG levels did not report statistically significant differences, but its role related to testosterone and estrogen levels during inflammation and its role in COVID-19 infection has to be further investigated, as it may explain the altered levels of TT and E2 [79].
Finally, our analysis confirms the impairment of sperm parameters reported by other studies. Literature shows that male patients who recovered from COVID-19 infection had worse semen parameters when compared to healthy controls for COVID-19 [80]. Our short-term analysis of seminal fluid demonstrates a wide variety of semen outcomes, ranging from azoospermia to normozoospermia. We found lower sperm concentration, total sperm count, and total sperm motility. Progressive sperm motility was not significantly different, but few studies have evaluated it. These findings may reveal important concerns for male fertility, as they could reduce the fertilization rate, both in natural pregnancies and in assisted reproductive techniques. Certainly, further studies are needed as a long-term follow-up period is missing and the long-term effects of COVID-19 infection have yet to be clarified, and the recovery time of sperm parameters. The results of our meta-analysis are summarized in Table 5.
The longest follow-up study assessing semen parameters was conducted by Hu et al. [81], who conducted an additional follow-up investigation on COVID-19 patients with a median recovery time of 177.5 days compared to age-matched healthy controls for sperm quality. After a 120-day recovery period, total sperm motility did not change, and sperm count had improved, while the total number of sperm in this group was impaired. Confirmation and investigation of the precise moment at which sperm quality started to increase require bigger cohort research with varying recovery times.
Similar investigations were conducted by Ma et al. [51] who evaluated the sperm quality and sex hormone profiles in 12 male COVID-19 patients whose median period of semen collection was 78.5 days from the onset of the disease. The findings basically showed that 119 male COVID-19 patients had reduced gonadal function and some patients had lower sperm motility and increased sperm DNA fraction percentages. The study by Holtmann et al. [48] found that COVID-19 patients had significantly lower sperm quality compared to the control group when there was a mean interval of 25.5 days between the end of symptoms and semen collection. Both investigations showed that male COVID-19 patients’ sperm quality began to drop during the early stages of recovery (related to the spermatogenesis cycle). Thus, it could be hypothesized that COVID-19 patients’ sperm quality increased following a recovery period of roughly 6 months.
Concerning the detection of SARS-CoV-2 mRNA in seminal fluid, literature searches are still controversial and therefore inconclusive [82]. Most of the studies, including our meta-analysis, have not confirmed the existence of the SARS-CoV-2 virus in the semen of male COVID-19 patients, while some studies have detected the virus RNA in the semen of COVID-19-infected patients [83,84,85]. These latter studies suggest that the SARS-CoV-2 virus may be able to enter the testis and colonize the semen. In severe clinical cases with a high viral load and the acute phase of the disease, the systemic inflammatory response allows the virus to spread across the blood testis barrier. Consequently, the infection could impair spermatogenesis and hormone secretion in COVID-19 patients, leading to male fertility impairment. More studies need to be done as it is unclear how long it takes to clear the virus from semen. Li et al. [86] reported for the first time the detection of SARS-CoV-2 in the semen of 38 patients with a severe form of COVID-19 and during the acute phase of the disease. Therefore, the stage of the disease may be an important factor to take into account. This condition may correspond to a higher blood viral load and, therefore, a greater possibility of crossing the blood testis barrier. Interestingly, the most recent study published on this topic aimed to develop a reliable methodology capable of detecting SARS-CoV2 mRNA in the seminal fluid. The authors developed a qualitative RT-PCR assay, which was controlled for PRM1 and PRM2 mRNAs. A positive control obtained by diluting the viral preparation from a SARS-CoV-2 panel was used. Despite the precise methodology, the assay was unable to detect the presence of SARS-CoV-2 mRNA in the seminal fluid of patients with mild and recovered COVID-19 [87]. To the best of our knowledge, all studies evaluating the presence of SARS-CoV-2 in the seminal fluid are listed in Table 6. The studies included in our meta-analysis are not listed as semen analysis was negative for the SARS-CoV-2 RNA in all of them.
There is a lack of information regarding reproductive outcomes in critical and severe disease states, in contrast to mild and moderate disease states. Also unreported is the connection between disease severity, hormone levels, and semen parameters. According to Gacci et al. [88], it may be suggested a link between the severity of COVID-19 and azoospermia, with 7.0% of cases of severe oligoasthenoteratozoospermia and 18.6% of cases of azoospermia observed in severe illness. Semen parameters have been seen to revert to normal levels 79 days after the fever. Most findings suggested that 3 months following COVID-19 recovery, the overall state of andrological health appeared to be unaffected. It can be presumed that no major long-term impairment and no sperm autoimmune response had occurred during a full spermatogenetic cycle from recovery.
Recent autopsy series have reported testicular pathological findings in patients with COVID-19. SARS-CoV-2 viral antigen was detected in Sertoli cells, Leydig cells, spermatids, and fibroblast cells in rete testis under electron microscopy [89]. Furthermore, viral particles were identified by immunohistochemistry in multiple testicular cells [90] and SARS-CoV2 RNA was found in testicular tissue [91]. The most frequent macroscopic findings were mild interstitial orchitis, hyperemia, interstitial edema, thickening of the basal membrane, thinning of the seminiferous tubules, increased apoptotic cells in the tubules, reduction of Leydig and Sertoli cells, and spermatogenesis impairment [91]. The microscopic series reported impaired spermatogenesis, interstitial infiltration macrophages, and leukocytes, tubular damage with swelling of Sertoli cells, vacuolation, and shedding [35, 89, 92]. This evidence confirms that the testis may be susceptible and vulnerable to COVID-19. The finding of ACE-2 and TMPRSS2 can explain the pathological aspects found in male patients who died from COVID-19 infection with the presence of SARS-CoV-2 at the testicular level. Consequently, spermatogenesis and hormone secretion in COVID-19 patients would be damaged by the virus, resulting in impaired male fertility [29] (Fig. 12). Thus, it may be suggested that in the assessment of males affected by COVID-19 and diagnosed with epididymal-orchitis, the examination of sperm parameters and hormone levels is advised [93].
Mechanisms by which SARS-CoV-2 might damage testicular function in the acute phase and after short-term follow-up. In the acute phase, mechanisms mediated by inflammation, such as the increase in pro-inflammatory cytokines, lead to a reduction of GnRH pulses and, consequently, of serum LH and FSH levels. This is also favored by the use of glucocorticoids to treat COVID-19. After recovery, the hypothalamic-pituitary axis function will be restored. SARS-CoV-2 is also able to directly infect the testis and damage Leydig and Sertoli cells, thus leading to decreased testosterone biosynthesis and impaired spermatogenesis associated with elevated LH and unchanged FSH levels, in the short-term follow-up. The ability of these cells to regain their function will allow recovery from primary hypogonadism in the long-term follow-up. FSH follicle-stimulating hormone, GnRH gonadotropin-releasing hormone, IL interleukin, LH luteinizing hormone, SARS-CoV-2 severe acute respiratory syndrome Coronavirus 2, TNFα tumor necrosis factor α
Strengths of the study
Several studies and meta-analyses have been conducted on this topic. Our study adds new perspectives to the existing literature. One of the strengths of our study is the thorough hormonal evaluation. Most studies reported only the levels of LH, FSH, and TT. A few studies included PRL, E2, and SHBG. These assessments could provide a more comprehensive understanding of the pathophysiology of SARS-CoV-2 infection and its short- and long-term consequences on the male reproductive system. Monitoring the baseline level of prolactin in male patients may help with the prognosis and clinical management of COVID-19 due to the high concentration of prolactin, which inhibits the HPG axis’ signaling [94]. E2 is an important sex hormone that originates from the conversion of testosterone conversion by aromatase, which is expressed mainly in testis adipose tissue, bone, and brain. Inflammation enhances aromatase activity and thus the conversion of testosterone to E2. Indeed, our meta-analysis demonstrates that E2 levels are significantly higher in patients with COVID-19 than in healthy control men. It needs to be further clarified whether this increase is a co-cause or consequence of COVID-19 and whether this has a prognostic impact. Some studies have found greater morbidity and mortality from COVID-19 in male patients than in females, so it can be assumed that estrogens have a protective role by modulating the inflammatory process. In this regard, a study conducted by Seeland et al. [95] suggested the protective role of estrogens against severe COVID-19 through the downregulation of the ACE2 receptor. The increase of estrogens could also be related to a decrease in the testosterone/E2 ratio for the transient state of primary hypogonadism developed as a consequence of the direct damage of the testicular epithelium by SARS-CoV-21. Although E2 could increase in patients with obesity, it must be considered that the present meta-analysis includes studies with homogenous populations in terms of age and BMI which did not show a significant difference between patients and controls, nor relevant heterogeneity that could alter the results. Thus, the increase in E2 is independent of the BMI.
The presence of impairment of testicular function during active infection is not surprising, since all infections in the acute phase are capable of interfering by various mechanisms with gonadotropin secretion and spermatogenesis. The current challenge is to understand whether SARS-CoV2 infection is able to depress testicular function in the long term, therefore independently of acute inflammatory processes. Our meta-analysis provides evidence on patients’ testicular function up to 80 days after infection and therefore has the potential to answer this question. To date, the longest time monitoring testicular function after recovery from COVID-19 was 90 days, a similar duration to that of the present meta-analysis. A recent uncontrolled multicenter study of eighty patients whose gonadal function was assessed 3 months after recovery from COVID-19 revealed an indirect and transient impairment of testicular function induced by SARS-CoV2 infection [80]. However, being uncontrolled, this study does not provide definite evidence of the impact of COVID-19 infection on testicular function after recovery, and further data are needed to clarify this issue.
Our analysis was conducted on a total of 1250 patients with an active or anamnestic infection of COVID-19 and 1232 healthy controls. A strength of our study is the selection criteria of the patient population since in all studies that we selected, the infection was confirmed by RT-PCR analysis on nasal or pharyngeal swabs and/or positive serology for IgA/IgA antibodies. Studies that included patients with presumed COVID-19 infection based on pulmonary computerized tomography results were not included. Therefore, our analysis proposes a homogeneous and representative sample of patients with SARS-CoV-2 infection.
Limitations of the study
Our analysis has some limitations. Firstly, this is a short-term evaluation of patients with active or anamnestic COVID-19 infection (from baseline to up to 80 days from infection), thus it does not provide information on long-term follow-up and possible long-term consequences of COVID-19 infection on the male urogenital tract, on the recovery times from hypogonadism, and the possible consequences of protracted hormonal alterations.
Although our study population was homogeneous in terms of age and BMI, not all studies recorded clinical symptoms of the infection, associated comorbidities, or smoking habits. Furthermore, the interference of intercurrent pharmacological treatments (i.e., antivirals, antibiotics, corticosteroids, etc.) cannot be excluded. Some of the patients included in our study were aged from 57 to 65 years, an age for which a progressive decline in sperm parameters is already known in the literature [96,97,98]. Another limit is represented by the different timing of semen sample collection compared to the moment in which the infection was contracted. Some studies [99] reported collections performed during the acute symptomatic phase, while others [100] required collections about 70–80 days after infection and therefore during the convalescence phase. It has been suggested that semen analysis should be performed 3 months after infection to see changes in sperm quality once a new cycle of spermatogenesis is completed. Finally, some parameters of sperm quality, such as sperm DNA fragmentation (SDF), were not included in our study, as few studies have evaluated this parameter. Evaluation of SDF could open new insights into the understanding of SARS-CoV-2 pathophysiology and new perspectives on its implications for reproductive biology [101].
Conclusion
The SARS-CoV-2 pandemic has spread worldwide since 2019, with varying degrees of severity and threat and a mortality rate that, in some countries, reaches up to 10% [102]. Controlling this epidemic is still challenging due to the emergence of new variants and the ability of the virus to spread and survive. Furthermore, research interest is moving toward understanding the long-term consequences of the virus on the various apparatuses of the human body. For this reason, we conducted the present meta-analysis to evaluate the effects of SARS-CoV-2 infection on male gonadal function, evaluating the hormonal profile and sperm parameters. The results demonstrated a negative effect of SARS-CoV-2 infection both on the hormonal and reproductive profiles, which were evaluated at up to 80 days from infection.
Even while recent research indicated that the negative effects of SARS-CoV-2 infection on semen quality may only be transient, the length of time after infection resolution before spermatogenesis is restored is another issue that is still up for debate [80]. A conclusion about these topics could be important for couples that are ART candidates, as it may be suggested to advise infertile couples to postpone parenthood research or ART procedures around 3 months after recovery from the infection to maximize their chances of getting pregnant, due to evidence of temporary alterations of sperm DNA integrity close to the recovery. Moreover, a conclusion regarding these issues could be crucial for men undergoing procedures to cryopreserve male gametes [103].
Data availability
Data will be made available to the editors of the journal for review or query upon request.
References
F. Wu, S. Zhao, B. Yu, Y.M. Chen, W. Wang, Z.G. Song et al. A new coronavirus associated with human respiratory disease in China. Nature 579(7798), 265–269 (2020)
L. Chen, Q. Li, D. Zheng, H. Jiang, Y. Wei, L. Zou et al. Correspondence: clinical characteristics of Covid-19 in Wuhan China. N. Engl. J. Med. 100, 1 (2020)
S. La Vignera, R. Cannarella, R.A. Condorelli, F. Torre, A. Aversa, A.E. Calogero, Sex-specific SARS-CoV2 mortality: among hormone-modulated ace2 expression, risk of venous thromboembolism and hypovitaminosis D. Int. J. Mol. Sci. 21(8), 5–10 (2020)
L. Marinelli, G. Beccuti, M. Zavattaro, S. Cagnina, I. Gesmundo, C. Bona et al. Testosterone as a biomarker of adverse clinical outcomes in SARS-CoV-2 pneumonia. Biomedicines 10(4), 1–11 (2022)
A. Alamo, C. De Luca, L.M. Mongioì, F. Barbagallo, R. Cannarella, S. La Vignera, A.E. Calogero, R.A. Condorelli, Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility. Biomedicines 8(7), 196 (2020). https://doi.org/10.3390/biomedicines8070196
K.E. Stanley, E. Thomas, M. Leaver, D. Wells, Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil. Steril. 114(1), 33–43 (2020). https://doi.org/10.1016/j.fertnstert.2020.05.001
A. Sansone, D. Mollaioli, G. Ciocca, E. Limoncin, E. Colonnello, W. Vena et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J. Endocrinol. Invest. 44(2), 223–231 (2021). https://doi.org/10.1007/s40618-020-01350-1
J. Xu, L. Qi, X. Chi, J. Yang, X. Wei, E. Gong et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol. Reprod. 74(2), 410–416 (2006)
M. Bendayan, F. Boitrelle, What could cause the long-term effects of COVID-19 on sperm parameters and male fertility? QJM 114(4), 287 (2021)
R. Channappanavar, C. Fett, M. Mack, P.P. Ten Eyck, S. Perlman, I. City et al. Sex-based differences in susceptibility to SARS-CoV infection. J. Immunol. 198(10), 319–335 (2018)
L. Lanser, F.R. Burkert, L. Thommes, A. Egger, G. Hoermann, S. Kaser et al. Testosterone deficiency is a risk factor for severe COVID-19. Front. Endocrinol. 12, 1–12 (2021)
M. Zaigham, A. Holmberg, M.L. Karlberg, O.K. Lindsjö, L. Jokubkiene, J. Sandblom et al. Intrauterine vertical SARS-CoV-2 infection: a case confirming transplacental transmission followed by divergence of the viral genome. BJOG Int. J. Obstet. Gynaecol. 128(8), 1388–1394 (2021)
M.Y. Valdespino-Vázquez, C.A. Helguera-Repetto, M. León-Juárez, O. Villavicencio-Carrisoza, A. Flores-Pliego, E.R. Moreno-Verduzco et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 93(7), 4480–4487 (2021)
G. Rastrelli, V. Di Stasi, F. Inglese, M. Beccaria, M. Garuti, D. Di Costanzo et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 9(1), 88–98 (2021)
S. Dhindsa, N. Zhang, M.J. McPhaul, Z. Wu, A.K. Ghoshal, E.C. Erlich, K. Mani, G.J. Randolph, J.R. Edwards, P.A. Mudd, A. Diwan, Association of Circulating Sex Hormones With Inflammation and Disease Severity in Patients With COVID-19. JAMA Netw Open 4(5), e2111398 (2021). https://doi.org/10.1001/jamanetworkopen.2021.11398
L. Kim, S. Garg, A. O’Halloran, M. Whitaker, H. Pham, E.J. Anderson et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 72(9), E206–E214 (2021)
F. Barbagallo, A.E. Calogero, R. Cannarella, R.A. Condorelli, L.M. Mongioì, A. Aversa et al. The testis in patients with COVID-19: virus reservoir or immunization resource? Transl. Androl. Urol. 9(5), 1897–1900 (2020)
M. Iwasaki, J. Saito, H. Zhao, A. Sakamoto, K. Hirota, D. Ma, Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: molecular mechanisms and implications. Inflammation 44(1), 13–34 (2021)
S. Çayan, M. Uğuz, B. Saylam, E. Akbay, Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. Aging Male 23(5), 1493–1503 (2021). https://doi.org/10.1080/13685538.2020.1807930
A.E. Cinislioglu, N. Cinislioglu, S.O. Demirdogen, E. Sam, F. Akkas, M.S. Altay et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: a prospective study. Andrology 10(1), 24–33 (2022)
M. Kadihasanoglu, S. Aktas, E. Yardimci, H. Aral, Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. (2020)
A.S. Patel, J.Y. Leong, L. Ramos, R. Ramasamy, Testosterone is a contraceptive and should not be used in men who desire fertility. World J. Mens Health 37(1), 45–54 (2019)
S. Okçelik, COVID-19 pneumonia causes lower testosterone levels. Andrologia 53(1), 1–4 (2021)
K. Selvaraj, S. Ravichandran, S. Krishnan, R.K. Radhakrishnan, N. Manickam, M. Kandasamy, Testicular atrophy and hypothalamic pathology in COVID-19: possibility of the incidence of male infertility and HPG axis abnormalities. Reprod. Sci. 28(10), 2735–2742 (2021)
H. Rosen, M.L. Jameel, A.L. Barkan, Dexamethasone suppresses gonadotropin-releasing hormone (GnRH) secretion and has direct pituitary effects in male rats: differential regulation of gnrh receptor and gonadotropin responses to GnRH. Endocrinology 122(6), 2873–2880 (1988)
L.F. Perez-Garcia, B. te Winkel, J.P. Carrizales, W. Bramer, S. Vorstenbosch, E. van Puijenbroek et al. Sexual function and reproduction can be impaired in men with rheumatic diseases: a systematic review. Semin. Arthritis Rheum. 50(3), 557–573 (2020). https://doi.org/10.1016/j.semarthrit.2020.02.002
S. Bank, S.K. De, B. Bankura, S. Maiti, M. Das, G.A. Khan, Ace/ace2 balance might be instrumental to explain the certain comorbidities leading to severe covid-19 cases. Biosci. Rep. 41(2), 41–46 (2021)
C. Fan, W. Lu, K. Li, Y. Ding, J. Wang, ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front. Med. 7, 1–9 (2021)
S. Colaco, K. Chhabria, D. Singh, A. Bhide, N. Singh, A. Singh et al. Expression map of entry receptors and infectivity factors for pan-coronaviruses in preimplantation and implantation stage human embryos. J. Assist. Reprod. Genet. 38(7), 1709–1720 (2021)
G. Mjaess, A. Karam, F. Aoun, S. Albisinni, T. Roumeguère, COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog. Urol. 30(10), 484–487 (2020). https://doi.org/10.1016/j.purol.2020.05.007
E. Taglauer, Y. Benarroch, K. Rop, E. Barnett, V. Sabharwal, C. Yarrington et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta 100, 69–74 (2020). https://doi.org/10.1016/j.placenta.2020.08.015
J. Qi, Y. Zhou, J. Hua, L. Zhang, J. Bian, B. Liu et al. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to SARS-CoV-2 infection. Int. J. Environ. Res. Public Health 18(1), 1–17 (2021)
L.M. Wong, G. Jiang, A plausible link of tmprss2/ace2/ar signaling to male mortality during the COVID-19 pandemic in the United States. Pathogens 10(11), 1–10 (2021)
D. Cui, Y. Liu, X. Jiang, C. Ding, L.C. Poon, H. Wang et al. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet. Gynecol. 57(2), 248–256 (2021)
N. Moghimi, B. Eslami Farsani, M. Ghadipasha, G.R. Mahmoudiasl, A. Piryaei, A. Aliaghaei et al. COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis 26(7–8), 415–430 (2021). https://doi.org/10.1007/s10495-021-01680-2
C. Massarotti, A. Garolla, E. Maccarini, P. Scaruffi, S. Stigliani, P. Anserini et al. SARS-CoV-2 in the semen: where does it come from? Andrology 9(1), 39–41 (2021)
S. Abdollahpour, S. Badiee Aval, T. Khadivzadeh, Do not neglect the Covid-19 transmission through sexual intercourse. J. Sex. Marital Ther. 47(7), 731–737 (2021). https://doi.org/10.1080/0092623X.2021.1938765
M.J. Perry, S. Arrington, L.M. Neumann, D. Carrell, C.N. Mores, It is currently unknown whether SARS-CoV-2 is viable in semen or whether COVID-19 damages spermatozoa. Andrology 9(1), 30–32 (2021)
A.M. Methley, S. Campbell, C. Chew-Graham, R. McNally, S. Cheraghi-Sohi, PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 14, 1 (2014)
X. Wan, W. Wang, J. Liu, T. Tong, Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14(1), 1–13 (2014)
J. Murray, D.P. Farrington, M.P. Eisner, Drawing conclusions about causes from systematic reviews of risk factors: the Cambridge Quality Checklists. J. Exp. Criminol. 5(1), 1–23 (2009)
T.-H. Guo, M.-Y. Sang, S. Bai, H. Ma, Y.-Y. Wan, X.-H. Jiang, Y.-W. Zhang, B. Xu, H. Chen, X.-Y. Zheng, S.-H. Luo, X.-F. Xie, C.-J. Gong, J.-P. Weng, Q.-H. Shi, Semen parameters in men recovered from COVID-19. Asian J. Androl. (2021). https://pubmed.ncbi.nlm.nih.gov/33975987/#:~:text=Thetotalspermcount%2Cspermconcentration%2Candnumberofmotileinthe22patientsexamined
T. Chen, D. Wu, H. Chen, W. Yan, D. Yang, G. Chen, K. Ma, D. Xu, H. Yu, H. Wang, T. Wang, W. Guo, J. Chen, C. Ding, X. Zhang, J. Huang, M. Han, S. Li, X. Luo, J. Zhao, Q. Ning, Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368, m1091 (2020). Erratum in: BMJ. 368, m1295 (2020). https://doi.org/10.1136/bmj.m1091
M.Z. Temiz, M.M. Dincer, I. Hacibey, R.O. Yazar, C. Celik, S.H. Kucuk et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia 53(2), 1–9 (2021)
J.C. Best, M. Kuchakulla, K. Khodamoradi, T.F.N. Lima, F.S. Frech, J. Achua et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J. Mens Health 39(3), 489–495 (2021)
Y. Ruan, B. Hu, Z. Liu, K. Liu, H. Jiang, H. Li et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology 9(1), 99–106 (2021)
H. Li, X. Xiao, J. Zhang, M.I. Zafar, C. Wu, Y. Long et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 28, 100604 (2020). https://doi.org/10.1016/j.eclinm.2020.100604
N. Holtmann, P. Edimiris, M. Andree, C. Doehmen, D. Baston-Buest, O. Adams et al. Assessment of SARS-CoV-2 in human semen—a cohort study. Fertil. Steril. 114(2), 233–238 (2020)
H. Piroozmanesh, E. Cheraghi, L. Naserpoor, M. Aghashahi, R. Jannatifar, The effect of COVID-19 infection on sperm quality and male fertility. Jentashapir J. Cell Mol. Biol. 12(2), e115390 (2021). https://doi.org/10.5812/jjcmb.115390
H. Xu, Z. Wang, C. Feng, W. Yu, Y. Chen, X. Zeng et al. Effects of SARS-CoV-2 infection on male sex-related hormones in recovering patients. Andrology 9(1), 107–114 (2021)
L. Ma, W. Xie, D. Li, L. Shi, G. Ye, Y. Mao et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J. Med. Virol. 93(1), 456–462 (2021). https://doi.org/10.1002/jmv.26259
L. Guo, S. Zhao, W. Li, Y. Wang, L. Li, S. Jiang et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology 9(1), 42–47 (2021)
M.M. Aboelnaga, A. Abdelrazek, N. Abdullah, M. El Shaer, Late impact of COVID-19 pneumonia on testosterone levels in recovered, post-hospitalized male patients. J. Endocrinol. Metab. 11(3–4), 76–82 (2021)
M. Camici, P. Zuppi, P. Lorenzini, L. Scarnecchia, C. Pinnetti, S. Cicalini et al. Role of testosterone in SARS-CoV-2 infection: a key pathogenic factor and a biomarker for severe pneumonia. Int. J. Infect. Dis. 108, 244–251 (2021)
A. Salonia, M. Pontillo, P. Capogrosso, S. Gregori, M. Tassara, L. Boeri et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology 9(4), 1043–1052 (2021)
Y. Pazir, T. Eroglu, A. Kose, T.B. Bulut, C. Genc, M. Kadihasanoglu, Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: a prospective cohort study. Andrologia 53(9), 1–6 (2021)
A. Sansone, D. Mollaioli, G. Ciocca, E. Colonnello, E. Limoncin, G. Balercia et al. “Mask up to keep it up”: preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology 9(4), 1053–1059 (2021)
A. Salonia, G. Corona, A. Giwercman, M. Maggi, S. Minhas, R.E. Nappi et al. SARS-CoV-2, testosterone and frailty in males (PROTEGGIMI): a multidimensional research project. Andrology 9(1), 19–22 (2021)
M. Schroeder, B. Schaumburg, Z. Mueller, A. Parplys, D. Jarczak, K. Roedl et al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerg. Microbes Infect. 10(1), 1807–1818 (2021)
P. Gupta, A. Choudhary, G. Gopal, R. Kumar, A. Kumar, P. Tiwari et al. Detection of SARS-CoV2 virus using the real-time reverse transcriptase polymerase chain reaction in semen and seminal plasma from men with active COVID-19 infection—a pilot study. Indian J. Urol. 37(4), 331–334 (2021)
R.E. Bridwell, D.R. Merrill, S.A. Griffith, J. Wray, J.J. Oliver, A coronavirus disease 2019 (COVID-19) patient with bilateral orchitis. Am. J. Emerg. Med. 42, 260.e3–260.e5 (2021)
A. La Marca, S. Busani, V. Donno, G. Guaraldi, G. Ligabue, M. Girardis, Testicular pain as an unusual presentation of COVID-19: a brief review of SARS-CoV-2 and the testis. Reprod. Biomed. Online 41(5), 903–906 (2020). https://doi.org/10.1016/j.rbmo.2020.07.017
C. Ediz, H.H. Tavukcu, S. Akan, Y.E. Kizilkan, A. Alcin, K. Oz et al. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int. J. Clin. Pr. 75(3), 1–5 (2021)
L.M. Mongioì, F. Barbagallo, R.A. Condorelli, R. Cannarella, A. Aversa, S. La Vignera et al. Possible long-term endocrine-metabolic complications in COVID-19: lesson from the SARS model. Endocrine 68(3), 467–470 (2020). https://doi.org/10.1007/s12020-020-02349-7
S. Wang, A. Zhang, Y. Pan, L. Liu, S. Niu, F. Zhang et al. Association between COVID-19 and male fertility: systematic review and meta-analysis of observational studies. World J. Mens. Health 40, 1–19 (2022)
G. Corona, W. Vena, A. Pizzocaro, F. Pallotti, D. Paoli, G. Rastrelli et al. Andrological effects of SARS-Cov-2 infection: a systematic review and meta-analysis. J. Endocrinol. Invest. 45(12), 2207–2219 (2022). https://doi.org/10.1007/s40618-022-01801-x
Y. Xie, M. Mirzaei, M.S. Kahrizi, A.M. Shabestari, S.M. Riahi, M. Farsimadan et al. SARS-CoV-2 effects on sperm parameters: a meta-analysis study. J. Assist. Reprod. Genet. 39(7), 1555–1563 (2022). https://doi.org/10.1007/s10815-022-02540-x
X. Chen, J. Ding, M. Liu, K. Xing, P. Ye, J. Min et al. A systemic review and meta-analysis of the effect of SARS-CoV-2 infection on sperm parameters. Research 2022, 1–13 (2022)
S.H. Russell, C.J. Small, S.A. Stanley, S. Franks, M.A. Ghatei, S.R. Bloom, The in vitro role of tumour necrosis factor-alpha and interleukin-6 in the hypothalamic-pituitary gonadal axis. J. Neuroendocrinol. 13(3), 296–301 (2001)
H. Watanobe, Y. Hayakawa, Hypothalamic interleukin-1β and tumor necrosis factor-α, but not interleukin-6, mediate the endotoxin-induced suppression of the reproductive axis in rats. Endocrinology 144(11), 4868–4875 (2003)
D.I. Spratt, J.R. Morton, R.S. Kramer, S.W. Mayo, C. Longcope, C.P.H. Vary, Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am. J. Physiol. Endocrinol. Metab. 291(3), 631–638 (2006)
M. Watanabe, D. Caruso, D. Tuccinardi, R. Risi, M. Zerunian, M. Polici et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 111, 154319 (2020). https://doi.org/10.1016/j.metabol.2020.154319
R. Singh, S.S. Rathore, H. Khan, S. Karale, Y. Chawla, K. Iqbal et al. Association of obesity with COVID-19 severity and mortality: an updated systemic review, meta-analysis, and meta-regression. Front. Endocrinol. 13(Jun), 1–18 (2022)
F. Barbagallo, R.A. Condorelli, L.M. Mongioì, R. Cannarella, L. Cimino, M.C. Magagnini et al. Molecular mechanisms underlying the relationship between obesity and male infertility. Metabolites 11(12), 840 (2021). https://doi.org/10.3390/metabo11120840
S. La Vignera, R. Cannarella, F. Galvano, A. Grillo, A. Aversa, L. Cimino et al. The ketogenic diet corrects metabolic hypogonadism and preserves pancreatic ß-cell function in overweight/obese men: a single-arm uncontrolled study. Endocrine 72(2), 392–399 (2021). https://doi.org/10.1007/s12020-020-02518-8
L.M. Mongioì, L. Cimino, R.A. Condorelli, M.C. Magagnini, F. Barbagallo, R. Cannarella et al. Effectiveness of a very low calorie ketogenic diet on testicular function in overweight/obese men. Nutrients 12(10), 1–11 (2020)
B. Kumar, M. Gopalakrishnan, M. Garg, P. Purohit, M. Banerjee, P. Sharma et al. Endocrine dysfunction among patients with COVID-19: a single-center experience from a tertiary hospital in India. Indian J. Endocrinol. Metab. 25(1), 14 (2021)
H.M. Al-Kuraishy, A.I. Al-Gareeb, M. Butnariu, G.E.S. Batiha, The crucial role of prolactin-lactogenic hormone in Covid-19. Mol. Cell Biochem. 477(5), 1381–1392 (2022). https://doi.org/10.1007/s11010-022-04381-9
I.E. Van Zeggeren, A. Boelen, D. Van De Beek, A.C. Heijboer, A.P.J. Vlaar, M.C. Brouwer, Sex steroid hormones are associated with mortality in COVID-19 patients: level of sex hormones in severe COVID-19. Medicine 100(34), E27072 (2021)
D. Paoli, F. Pallotti, A. Anzuini, S. Bianchini, L. Caponecchia, A. Carraro et al. Male reproductive health after 3 months from SARS-CoV-2 infection: a multicentric study. J. Endocrinol. Invest. 46(1), 89–101 (2023). https://doi.org/10.1007/s40618-022-01887-3
B. Hu, K. Liu, Y. Ruan, X. Wei, Y. Wu, H. Feng et al. Evaluation of mid- and long-term impact of COVID-19 on male fertility through evaluating semen parameters. Transl. Androl. Urol. 11(2), 159–167 (2022)
S.K. Patel, M. Pathak, R. Sah, A. Kumar, Y.S. Malik, A.J. Rodríguez-Morales et al. Is sexual route a matter of concern for the SARS-CoV-2/COVID-19? Arch. Med. Res. 51(7), 745–746 (2020)
L.J. Purpura, J. Alukal, A.M. Chong, L. Liu, A. Cantos, J. Shah et al. SARS-CoV-2 RNA shedding in semen and oligozoospermia of patient with severe coronavirus disease 11 weeks after infection. Emerg. Infect. Dis. 28(1), 196–200 (2022)
B. Saylam, M. Uguz, M. Yarpuzlu, O. Efesoy, E. Akbay, S. Çayan, The presence of SARS-CoV-2 virus in semen samples of patients with COVID-19 pneumonia. Andrologia 53(8), e14145 (2021). https://doi.org/10.1111/and.14145
B. Machado, G.B. Barra, N. Scherzer, J. Massey, H. dos Santos Luz, R.H. Jacomo et al. Presence of SARS-CoV-2 RNA in semen—cohort study in the United States COVID-19 positive patients. Infect. Dis. Rep. 13(1), 96–101 (2021)
D. Li, M. Jin, P. Bao, W. Zhao, S. Zhang, Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw. Open 3(5), e208292 (2020)
D. Paoli, F. Pallotti, G. Nigro, L. Mazzuti, M.N. Hirsch, M.B. Valli et al. Molecular diagnosis of SARS-CoV-2 in seminal fluid. J. Endocrinol. Invest. 44(12), 2675–2684 (2021). https://doi.org/10.1007/s40618-021-01580-x
M. Gacci, M. Coppi, E. Baldi, A. Sebastianelli, C. Zaccaro, S. Morselli et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum. Reprod. 36(6), 1520–1529 (2021. https://academic.oup.com/humrep/article/36/6/1520/6125160
A.N. Duarte-Neto, T.A. Teixeira, E.G. Caldini, C.T. Kanamura, M.S. Gomes-Gouvêa, A.B.G. dos Santos et al. Testicular pathology in fatal COVID-19: a descriptive autopsy study. Andrology 10(1), 13–23 (2022)
M. Yang, S. Chen, B. Huang, J.M. Zhong, H. Su, Y.J. Chen et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur. Urol. Focus 6(5), 1124–1129 (2020). https://doi.org/10.1016/j.euf.2020.05.009
X. Ma, C. Guan, R. Chen, Y. Wang, S. Feng, R. Wang et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol. Immunol. 18(2), 487–489 (2021). https://doi.org/10.1038/s41423-020-00604-5
J.K. Achua, K.Y. Chu, E. Ibrahim, K. Khodamoradi, K.S. Delma, O.A. Iakymenko et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J. Mens Health 39(1), 65–74 (2020)
P. Dai, F. Qiao, Y. Chen, D.Y.L. Chan, H.C.H. Yim, K.L. Fok et al. SARS-CoV-2 and male infertility: from short- to long-term impacts. J. Endocrinol. Invest. 0123456789 (2023). https://doi.org/10.1007/s40618-023-02055-x
V. Nargund, Effects of psychological stress on male fertility. Nat. Rev. Urol. 12, 373–382 (2015)
U. Seeland, F. Coluzzi, M. Simmaco, C. Mura, P.E. Bourne, M. Heiland et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 18(1), 1–9 (2020)
I.D. Harris, C. Fronczak, L. Roth, R.B. Meacham, Fertility and the aging male. Rev. Urol. 13(4), e184–e190 (2011. ; http://www.ncbi.nlm.nih.gov/pubmed/22232567%0A
V. Pino, A. Sanz, N. Valdés, J. Crosby, A. Mackenna, The effects of aging on semen parameters and sperm DNA fragmentation. J. Bras. Reprod. Assist. 24(1), 82–6 (2020)
R.A. Condorelli, S. La Vignera, F. Barbagallo, A. Alamo, L.M. Mongioì, R. Cannarella et al. Bio-functional sperm parameters: does age matter? Front. Endocrinol. 11, 1–7 (2020)
G. Anifandis, C.I. Messini, M. Simopoulou, G. Sveronis, A. Garas, A. Daponte et al. SARS-CoV-2 vs. human gametes, embryos and cryopreservation. Syst. Biol. Reprod. Med. 67(4), 260–269 (2021). https://doi.org/10.1080/19396368.2021.1922537
F. Pan, X. Xiao, J. Guo, Y. Song, H. Li, D.P. Patel et al. No evidence of severe acute respiratory syndrome–coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil. Steril. 113(6), 1135–1139 (2020). https://doi.org/10.1016/j.fertnstert.2020.04.024
F.M. Falahieh, M. Zarabadipour, M. Mirani, M. Abdiyan, M. DInparvar, H. Alizadeh et al. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod. Fertil. Dev. 33(12), 683–690 (2021)
No Title [Internet]. https://coronavirus.jhu.edu/data/mortality
V. Rago, A. Perri, SARS-CoV-2 infection and the male reproductive system: a brief review. Life (Basel). 13(2), 586 (2023). https://doi.org/10.3390/life13020586
A.P. Sharma, S. Sahoo, K. Goyal, A. Chandna, S. Kirubanandhan, V. Sharma et al. Absence of SARS-CoV-2 infection in the semen of men recovering from COVID-19 infection: an exploratory study and review of literature. Andrologia 53(8), 1–9 (2021)
C.A. Burke, A. Skytte, S. Kasiri, D. Howell, Z.P. Patel, M.P. Trolice, S.J. Parekattil, S.F. Michael, L.M. Paul, A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J. Assist. Reprod. Genet.(2021). https://www.scopus.com/record/display.uri?eid=2-s2.0-85104904086&doi=10.1007%2Fs10815-021-02119-y&origin=inward&txGid=1330be3b9802f1195c6b50658042fbc2
D. Paoli, F. Pallotti, O. Turriziani, L. Mazzuti, G. Antonelli, A. Lenzi et al. SARS-CoV-2 presence in seminal fluid: myth or reality. Andrology 9(1), 23–26 (2021)
B. Kayaaslan, G. Korukluoglu, I. Hasanoglu, A.K. Kalem, F. Eser, E. Akinci et al. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol. Int. 104(9–10), 678–683 (2020)
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
R.C. and A.E.C. conceived the study; R.C. and M.M. made the literature search; R.C. and M.M. extracted and analyzed the data; M.M. wrote the draft; R.C., A.C., V.B. and A.E.C. reviewed the article; S.L.V. and R.A.C. supervised the study; R.C. and A.E.C. are the project managers. All authors approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cannarella, R., Marino, M., Crafa, A. et al. Impact of COVID-19 on testicular function: a systematic review and meta-analysis. Endocrine 85, 44–66 (2024). https://doi.org/10.1007/s12020-024-03705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-024-03705-7