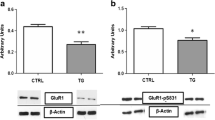
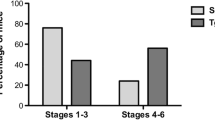
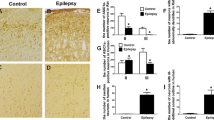
Abstract
Transgenic mice overexpressing spermine oxidase (SMO) in the cerebral cortex (Dach-SMO mice) showed increased vulnerability to excitotoxic brain injury and kainate-induced epileptic seizures. To investigate the mechanisms by which SMO overexpression leads to increased susceptibility to kainate excitotoxicity and seizure, in the cerebral cortex of Dach-SMO and control mice we assessed markers for astrocyte proliferation and neuron loss, and the ability of kainate to evoke glutamate release from nerve terminals and astrocyte processes. Moreover, we assessed a possible role of astrocytes in an in vitro model of epileptic-like activity in combined cortico-hippocampal slices recorded with a multi-electrode array device. In parallel, as the brain is a major metabolizer of oxygen and yet has relatively feeble protective antioxidant mechanisms, we analyzed the oxidative status of the cerebral cortex of both SMO-overexpressing and control mice by evaluating enzymatic and non-enzymatic scavengers such as metallothioneins. The main findings in the cerebral cortex of Dach-SMO mice as compared to controls are the following: astrocyte activation and neuron loss; increased oxidative stress and activation of defense mechanisms involving both neurons and astrocytes; increased susceptibility to kainate-evoked cortical epileptogenic activity, dependent on astrocyte function; appearance of a glutamate-releasing response to kainate from astrocyte processes due to activation of Ca2+-permeable AMPA receptors in Dach-SMO mice. We conclude that reactive astrocytosis and activation of glutamate release from astrocyte processes might contribute, together with increased reactive oxygen species production, to the vulnerability to kainate excitotoxicity in Dach-SMO mice. This mouse model with a deregulated polyamine metabolism would shed light on roles for astrocytes in increasing vulnerability to excitotoxic neuron injury.








Similar content being viewed by others
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Alloisio, S., Cervetto, C., Passalacqua, M., Barbieri, R., Maura, G., Nobile, M., & Marcoli, M. (2008). Functional evidence for presynaptic P2X(7) receptors in adult rat cerebrocortical nerve terminals. FEBS Letters, 582, 3948–3953.
Araque, A., Parpura, V., Sanzgiri, R. P., & Haydon, P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22, 208–215.
Aschner, M. (2006). The functional significance of brain metallothioneins. FASEB Journal, 10, 1129–1136.
Bellelli, A., Cavallo, S., Nicolini, L., Cervelli, M., Bianchi, M., Mariottini, P., et al. (2004). Mouse spermine oxidase: A model of the catalytic cycle and its inhibition by N,N1-bis(2,3-butadienyl)-1,4-butanediamine. Biochemical and Biophysical Research Communications, 322, 1–8.
Bernardinelli, Y., Muller, D., & Nikonenko, I. (2014). Astrocyte-synapse structural plasticity. Neural Plasticity. doi:10.1155/2014/232105.
Berretta, N., Ledonne, A., Mango, D., Bernardi, G., & Mercuri, N. B. (2012). Hippocampus vs. entorhinal cortex decoupling by an NR2 subunit-specific block of NMDA receptors in a rat in vitro model of temporal lobe epilepsy. Epilepsia, 53, e80–e84.
Birben, E., Murat Sahiner, U., Sackesen, C., Erzurum, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. The World Allergy Organization Journal, 5, 9–19.
Bleakman, D., & Lodge, D. (1998). Neuropharmacology of AMPA and kainate receptors. Neuropharmacology, 37, 1187–1204.
Boulter, J., Hollmann, M., O’Shea-Greenfield, A., Hartley, M., Deneris, E., Maron, C., & Heinemann, S. (1990). Molecular cloning and functional expression of glutamate receptor subunit genes. Science, 249, 1033–1037.
Bowie, D., & Mayer, M. L. (1995). Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron, 15, 453–462.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Capone, C., Cervelli, M., Angelucci, E., Colasanti, M., Macone, A., Mariottini, P., & Persichini, T. (2013). A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radical Biology and Medicine, 63, 99–107.
Carriedo, S. G., Yin, H. Z., Sensi, S. L., & Weiss, J. H. (1998). Rapid Ca2+ entry through Ca2+-permeable AMPA/Kainate channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. Journal of Neuroscience, 18, 7727–7738.
Cervelli, M., Angelucci, E., Germani, F., Amendola, R., & Mariottini, P. (2014). Inflammation, carcinogenesis and neurodegeneration studies in transgenic animal models for polyamine research. Amino Acids, 46, 521–530.
Cervelli, M., Bellavia, G., D’Amelio, M., Cavallucci, V., Moreno, S., Berger, J., et al. (2013a). A new transgenic mouse model for studying the neurotoxicity of spermine oxidase dosage in the response to excitotoxic injury. PLoS One, 8, e64810.
Cervelli, M., Bellavia, G., Fratini, E., Amendola, R., Polticelli, F., Barba, M., et al. (2010). Spermine oxidase (SMO) activity in breast tumor tissues and biochemical analysis of the anticancer spermine analogues BENSpm and CPENSpm. BMC Cancer, 10, 555.
Cervelli, M., Polticelli, F., Federico, R., & Mariottini, P. (2003). Heterologous expression and characterization of mouse spermine oxidase. Journal of Biological Chemistry, 278, 5271–5276.
Cervelli, M., Salvi, D., Polticelli, F., Amendola, R., & Mariottini, P. (2013b). Structure-function relationships in the evolutionary framework of spermine oxidase. Journal of Molecular Evolution, 76, 365–370.
Cervetto, C., Alloisio, S., Frattaroli, D., Mazzotta, M. C., Milanese, M., Gavazzo, P., et al. (2013a). The P2X7 receptor as a route for non-exocytotic glutamate release: Dependence on the carboxyl tail. Journal of Neurochemistry, 124, 821–831. doi:10.1111/jnc.1214.
Cervetto, C., Frattaroli, D., Maura, G., & Marcoli, M. (2013b). Motor neuron dysfunction in a mouse model of ALS: Gender-dependent effect of P2X7 antagonism. Toxicology, 311, 69–77.
Cervetto, C., Maura, G., & Marcoli, M. (2010). Inhibition of presynaptic release-facilitatory kainate autoreceptors by extracellular cyclic GMP. Journal of Pharmacology and Experimental Therapeutics, 332, 210–219.
Dogan, A., Rao, A. M., Hatcher, J., Rao, V. L., Baskaya, M. K., & Dempsey, R. J. (1999). Effects of MDL 72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. Journal of Neurochemistry, 72, 765–770.
Domin, H., Kajta, M., & Smialowska, M. (2006). Neuroprotective effects of MTEP, a selective mGluR5 antagonist and neuropeptide Y on the kainate-induced toxicity in primary neuronal cultures. Pharmacological Reports, 58, 846–858.
Dugan, L. L., Bruno, V. M. G., Amagasu, S. M., & Giffard, R. G. (1995). Glia modulate the response of murine cortical neurons to excitotoxicity: Glia exacerbate AMPA neurotoxicity. Journal of Neuroscience, 15, 4545–4555.
Ebadi, M., Brown-Borg, H., El Refaey, H., Singh, B. B., Garrett, S., Shavali, S., & Sharma, S. K. (2005). Metallothionein-mediated neuroprotection in genetically engineered mouse models of Parkinson’s disease. Molecular Brain Research, 134, 67–75.
Fellin, T., & Carmignoto, G. (2004). Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. Journal of Physiology, 559, 3–15.
Fleidervish, I. A., Libman, L., Katz, E., & Gutnick, M. J. (2008). Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proceedings of the National Academy of Sciences of the United States of America, 105, 18994–18999.
Fonnum, F., Johnsen, A., & Hassel, B. (1997). Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia, 21, 106–113.
Gallo, V., & Ghiani, C. A. (2000). Glutamate receptors in glia: New cells, new inputs and new functions. Trends in Pharmacological Sciences, 21, 252–258.
Gong, Y. H., & Elliott, J. L. (2000). Metallothionein expression is altered in a transgenic murine model of familial amyotrophic lateral sclerosis. Experimental Neurology, 162, 27–36.
Hidalgo, J., Aschner, M., Zatta, P., & Vasák, M. (2001). Roles of the metallothionein family of proteins in the central nervous system. Brain Research Bulletin, 55, 133–145.
Huang, T. F., & Huang, L. L. (1990). Effect of mannitol, n-2-mercaptopropionyl glycine and sodium nitroprusside on EEG recovery following cerebral ischemia and reperfusion in the rat. Chinese Journal of Physiology, 33, 121–129.
Iannicola, C., Moreno, S., Oliverio, S., Nardacci, R., Ciofi-Luzzatto, A., & Piacentini, M. (2000). Early alterations in gene expression and cell morphology in a mouse model of Huntington's disease. Journal of Neurochemistry, 75, 830–839.
Ivanova, S., Batliwalla, F., Mocco, J., Kiss, S., Huang, J., Mack, W., et al. (2002). Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proceedings of the National Academy of Sciences of the United States of America, 99, 5579–5584.
Ivanova, S., Botchkina, G. I., Al-Abed, Y., Meistrell, M, I. I. I., Batliwalla, F., Dubinsky, J. M., et al. (1998). Cerebral ischemia enhances polyamine oxidation: Identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. Journal of Experimental Medicine, 188, 327–340.
Jones, R. S., & Heinemann, U. (1988). Synaptic and intrinsic responses of medical entorhinal cortical cells in normal and magnesium-free medium in vitro. Journal of Neurophysiology, 59, 1476–1496.
Kamboj, S. K., Swanson, G. T., & Cull-Candy, S. G. (1995). Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. Journal of Physiology, 486, 297–303.
Koh, D. S., Burnashev, N., & Jonas, P. (1995). Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. Journal of Physiology, 486, 305–312.
Koike, M., Iino, M., & Ozawa, S. (1997). Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neuroscience Research, 29, 27–36.
Kwak, S. H., & Weiss, J. H. (2006). Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Current Opinion in Neurobiology, 16, 281–287.
Lanza, C., Morando, S., Voci, A., Canesi, L., Principato, M. C., Serpero, L. D., et al. (2009). Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. Journal of Neurochemistry, 110, 1674–1684.
Lanza, C., Raimondo, S., Vergani, L., Catena, N., Sénès, F., Tos, P., & Geuna, S. (2012). Expression of antioxidant molecules after peripheral nerve injury and regeneration. Journal of Neuroscience Research, 90, 842–848.
Lee, D. Z., Chung, J. M., Chung, K., & Kang, M. G. (2012). Reactive oxygen species (ROS) modulate AMPA receptor phosphorylation and cell-surface localization in concert with pain-related behavior. Pain, 153, 1905–1915.
Lerma, J., Paternain, A. V., Naranjo, J. R., & MellstrSm, B. (1993). Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America, 90, 11688–11692.
Li, W., Yuan, X. M., Ivanova, S., Tracey, K. J., Eaton, J. W., & Brunk, U. T. (2003). 3-aminopropanal, formed during cerebral ischaemia, is a potent lysosomotropic neurotoxin. Biochemical Journal, 371, 429–436.
Lipton, S. A., & Rosenberg, P. A. (1994). Excitatory amino acids as a final common pathway for neurologic disorders. New England Journal of Medicine, 330, 613–622.
Marcoli, M., Cervetto, C., Paluzzi, P., Guarnieri, S., Alloisio, S., Thellung, S., et al. (2008). P2X7 presynaptic receptors in adult rat cerebrocortical nerve terminals: A role in ATP-induced glutamate release. Journal of Neurochemistry, 105, 2330–2342.
Mastroberardino, P. G., Iannicola, C., Nardacci, R., Bernassola, F., De Laurenzi, V., Melino, G., et al. (2002). Tissue transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington's disease. Cell Death & Differentiation, 9, 873–880.
Nakamura, Y., Iga, K., Shibata, T., Shudo, M., & Kataoka, K. (1993). Glial plasmalemmal vesicles: A subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia, 9, 48–56.
Nilsen, A., & England, P. M. (2007). A subtype-selective, use-dependent inhibitor of native AMPA receptors. Journal of the American Chemical Society, 129, 4902–4903.
Park, M. H., & Igarashi, K. (2013). Polyamines and their metabolites as diagnostic markers of human diseases. Biomolecules & Therapeutics, 21, 1–9.
Parpura, V., Baker, B. J., Jeras, M., & Zorec, R. (2010). Regulated exocytosis in astrocytic signal integration. Neurochemistry International, 57, 451–459.
Partin, K. M., Patneau, D. K., Winters, C. A., Mayer, M. L., & Buonanno, A. (1993). Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron, 11, 1069–1082.
Pascual, O., Ben Achour, S., Rostaing, P., Triller, A., & Bessis, A. (2012). Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America, 109, E197–E205.
Paternain, A. V., Morales, M., & Lerma, J. (1995). Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron, 14, 185–189.
Penkowa, M. (2006). Metallothioneins are multipurpose neuroprotectants during brain pathology. FEBS Journal, 273, 1857–1870.
Poljsak, B., Šuput, D., & Milisav, I. (2013). Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Medicine and Cellular Longevity. doi:10.1155/2013/956792.
Polticelli, F., Salvi, D., Mariottini, P., Amendola, R., & Cervelli, M. (2012). Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evolutionary Biology, 12, 90.
Popa-Wagner, A., Mitran, S., Sivanesan, S., Chang, E., & Buga, A. M. (2013). ROS and brain diseases: The good, the bad, and the ugly. Oxidative Medicine and Cellular Longevity, 2013, Article ID 963520.
Rakhade, S. N., Fitzgerald, E. F., Klein, P. M., Zhou, C., Sun, H., Huganir, R. L., & Jensen, F. E. (2012). Glutamate receptor 1 phosphorylation at serine 831 and 845 modulates seizure susceptibility and hippocampal hyperexcitability after early life seizures. Journal of Neuroscience, 32, 17800–17812.
Rea, G., Bocedi, A., & Cervelli, M. (2004). Question: What is the biological function of the polyamines? IUBMB Life, 56, 167–169.
Rossi, D. J., Brady, J. D., & Mohr, C. (2007). Astrocyte metabolism and signaling during brain ischemia. Nature Neuroscience, 10, 1377–1386.
Saggu, H., Cooksey, J., Dexter, D., Wells, F. R., Lees, A., Jenner, P., & Marsden, C. D. (1989). A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. Journal of Neurochemistry, 53, 692–697.
Sofroniew, M. V. (2005). Reactive astrocytes in neural repair and protection. Neuroscientist, 11, 400–407.
Sofroniew, M. V., & Vinters, H. V. (2010). Astrocytes: Biology and pathology. Acta Neuropathologica, 119, 7–35.
Song, I., & Huganir, R. L. (2002). Regulation of AMPA receptors during synaptic plasticity. Trends in Neurosciences, 25, 578–588.
Stanfield, P. R., & Sutcliffe, M. J. (2003). Spermine is fit to block inward rectifier (Kir) channels. Journal of General Physiology, 122, 481–484.
Stigliani, S., Zappettini, S., Raiteri, L., Passalacqua, M., Melloni, E., Venturi, C., et al. (2006). Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. Journal of Neurochemistry, 96, 656–668.
Theodosis, D. T., Poulain, D. A., & Oliet, S. H. R. (2008). Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiological Reviews, 88, 983–1008.
Tsubokawa, H., Oguro, K., Masuzawa, T., Nakaima, T., & Kawai, N. (1995). Effects of spider toxin and its analogue on glutamate-activated currents in the hippocampal CA1 neuron after ischemia. Journal of Neurophysiology, 74, 218–225.
Uccelli, A., Milanese, M., Principato, M. C., Morando, S., Bonifacino, T., Vergani, L., et al. (2012). Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Molecular Medicine, 18, 794–804.
Vergani, L., Lanza, C., Rivaro, P., & Voci, A. (2011). Metals, metallothioneins and oxidative stress in blood of autistic children. Research in Autism Spectrum Disorders, 5, 286–293.
Vergani, L., Mascetti, G., & Nicolini, C. (1998). Effects of polyamines on higher-order folding of in situ chromatin. Molecular Biology Reports, 25, 237–244.
Wang, D. D., & Bordey, A. (2008). The astrocyte odyssey. Progress in Neurobiology, 86, 342–367.
Weiss, J. H. (2011). Ca2+ permeable AMPA channels in diseases of the nervous system. Frontiers in Molecular Neuroscience, 4, 42.
Wiechelman, K. J., Braun, R. D., & Fitzpatrick, J. D. (1988). Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Analytical Biochemistry, 175, 231–237.
Wilding, T. J., & Huettner, J. E. (1995). Differential antagonism of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Molecular Pharmacology, 47, 582–587.
Williams, K. (1997). Interactions of polyamines with ion channels. Biochemical Journal, 325, 289–297.
Wong, L. A., & Mayer, M. L. (1993). Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Molecular Pharmacology, 44, 504–510.
Wood, P. L., Khan, M. A., Kulow, S. R., Mahmood, S. A., & Moskal, J. R. (2006a). Neurotoxicity of reactive aldehydes: The concept of “aldehyde load” as demonstrated by neuroprotection with hydroxylamines. Brain Research, 1095, 190–199.
Wood, P. L., Khan, M. A., Moskal, J. R., Todd, K. G., Tanay, V. A. M. I., & Baker, G. (2006b). Aldehyde load in ischemia–reperfusion brain injury: Neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Research, 1122, 184–190.
Yang, D. S., Kumar, A., Stavrides, P., Peterson, J., Peterhoff, C. M., Pawlik, M., et al. (2008). Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer's disease. American Journal of Pathology, 173, 665–681.
Zhang, M., Caragine, T., Wang, H., Cohen, P. S., Botchkina, G., Soda, K., et al. (1997). Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. Journal of Experimental Medicine, 185, 1759–1768.
Zhang, X.-M., & Zhu, J. (2011). Kainic acid-induced neurotoxicity: Targeting glial responses and glia-derived cytokines. Current Neuropharmacology, 9, 388–398.
Acknowledgments
C.C. and M.M. thank the University of Genova for financial support; M.C. and P.M. wish to thank the University of Roma Tre for financial support. The authors acknowledge the excellent service of the mouse facility, University of Tor Vergata, Rome (I).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standard
All applicable international and national guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Cervetto, C., Vergani, L., Passalacqua, M. et al. Astrocyte-Dependent Vulnerability to Excitotoxicity in Spermine Oxidase-Overexpressing Mouse. Neuromol Med 18, 50–68 (2016). https://doi.org/10.1007/s12017-015-8377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-015-8377-3