Abstract
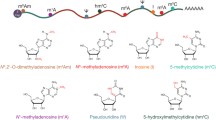
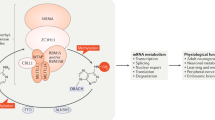
Precise regulation of transcriptome modulates several vital aspects in an organism that includes gene expression, cellular activities and development, and its perturbation ensuing pathological conditions. Around 150 post-transcriptional modifications of RNA have been identified till date, which are evolutionarily conserved and likewise prevalent across RNA classes including messenger RNA (mRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), and detected less frequently in small nuclear RNA (snRNA) and microRNAs (miRNA). Among the RNA modifications documented, N6-methyladenosine (m6A) is the best characterised till date. Also, N1-methyladenosine (m1A), 5-methylcytosine (m5C) and pseudouridine (Ψ) are some of the other prominent modifications detected in coding and non-coding RNAs. “Epitranscriptome”, ensemble of these post-transcriptional RNA modifications, precisely coordinates gene expression and biological processes. Current literatures suggest the critical involvement of epitranscriptomics in several organisms during early development, contributing to cell fate specification and physiology. Indeed, epitranscriptomics similar to DNA epigenetics involves combinatorial dynamics provided by modified RNA molecules and associated protein complexes, which function as “writers”, “erasers” and “readers” of these modifications. A novel code orchestrating gene expression during cell fate determination is generated by the coordinated effects of different classes of modified RNAs and its regulator proteins. In this review, we summarize the current knowhow on N6-methyladenosine (m6A), 5-methylcytosine (m5C) and pseudouridine (ψ) modifications in RNA, the associated regulator proteins and enumerate how the epitranscriptomic regulations are involved in cell fate determination.





Similar content being viewed by others
References
Li, S., & Mason, C. E. (2014). The pivotal regulatory landscape of RNA modifications. Annual Review of Genomics and Human Genetics, 15, 127–150.
Frye, M., Jaffrey, S. R., Pan, T., Rechavi, G., & Suzuki, T. (2016). RNA modifications: What have we learned and where are we headed? Nature Reviews Genetics, 17(6), 365–372.
Helm, M., & Motorin, Y. (2017). Detecting RNA modifications in the epitranscriptome: Predict and validate. Nature Reviews Genetics, 18(5), 275–291.
Cohn, W. E., & Volkin, E. (1951). Nucleoside-5′-phosphates from ribonucleic acid. Nature, 167, 483–484.
Roundtree, I. A., Evans, M. E., Pan, T., & He, C. (2017). Dynamic RNA modifications in gene expression regulation. Cell, 169(7), 1187–1200.
Schaefer, M., Pollex, T., Hanna, K., Tuorto, F., Meusburger, M., Helm, M., & Lyko, F. (2010). RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes and Development, 24(15), 1590–1595.
Hussain, S., Sajini, A. A., Blanco, S., Dietmann, S., Lombard, P., Sugimoto, Y., Paramor, M., Gleeson, J. G., Odom, D. T., Ule, J., & Frye, M. (2013). NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Reports, 4(2), 255–261.
Peifer, C., Sharma, S., Watzinger, P., Lamberth, S., Kötter, P., & Entian, K. D. (2013). Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Research, 41(2), 1151–1163.
Guy, M. P., & Phizicky, E. M. (2014). Two-subunit enzymes involved in eukaryotic post-transcriptional tRNA modification. RNA Biology, 11(12), 1608–1618.
Oerum, S., Dégut, C., Barraud, P., & Tisné, C. (2017). m1A post-transcriptional modification in tRNAs. Biomolecules, 7(1), 20.
Dominissini, D., Nachtergaele, S., Moshitch-Moshkovitz, S., Peer, E., Kol, N., Ben-Haim, M. S., Dai, Q., Di Segni, A., Salmon-Divon, M., & Clark, W. C. (2016). The dynamic N 1-methyladenosine methylome in eukaryotic messenger RNA. Nature, 530(7591), 441–446.
Li, X., Xiong, X., Wang, K., Wang, L., Shu, X., Ma, S., & Yi, C. (2016). Transcriptome-wide mapping reveals reversible and dynamic N 1-methyladenosine methylome. Nature Chemical Biology, 12(5), 311–316.
Zhao, B. S., Roundtree, I. A., & He, C. (2017). Post-transcriptional gene regulation by mRNA modifications. Nature Reviews Molecular Cell Biology, 18(1), 31–42.
Meyer, K. D., Saletore, Y., Zumbo, P., Elemento, O., Mason, C. E., & Jaffrey, S. R. (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell, 149(7), 1635–1646.
Batista, P. J., Molinie, B., Wang, J., Qu, K., Zhang, J., Li, L., Bouley, D. M., Lujan, E., Haddad, B., Daneshvar, K., Carter, A. C., Flynn, R. A., Zhou, C., Lim, K. S., Dedon, P., Wernig, M. A., Mullen, C., Xing, Y., Giallourakis, C. C., & Chang, H. Y. (2014). M(6)a RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell, 15(6), 707–719.
Liu, N., & Pan, T. (2016). N 6-methyladenosine–encoded epitranscriptomics. Nature Structural & Molecular Biology, 23(2), 98–102.
Hsu, P. J., Shi, H., & He, C. (2017). Epitranscriptomic influences on development and disease. Genome Biology, 18(1), 197.
Dunin-Horkawicz, S., Czerwoniec, A., Gajda, M. J., Feder, M., Grosjean, H., & Bujnicki, J. M. (2006). MODOMICS: A database of RNA modification pathways. Nucleic Acids Research, 34, D145–D149.
Boccaletto, P., Machnicka, M. A., Purta, E., Piatkowski, P., Baginski, B., Wirecki, T. K., de Crecy-Lagard, V., Ross, R., Limbach, P. A., Kotter, A., Helm, M., & Bujnicki, J. M. (2018). MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Research, 46(D1), D303–D307.
Sanchez-Vasquez, E., Alata Jimenez, N., Vazquez, N. A., & Strobl-Mazzulla, P. H. (2018). Emerging role of dynamic RNA modifications during animal development. Mechanisms of Development, 154, 24–32.
Dubin, D. T., & Taylor, R. H. (1975). The methylation state of poly A-containing-messenger RNA from cultured hamster cells. Nucleic Acids Research, 2(10), 1653–1668.
Peer, E., Rechavi, G., & Dominissini, D. (2017). Epitranscriptomics: Regulation of mRNA metabolism through modifications. Current Opinion in Chemical Biology, 41, 93–98.
Angelova, M. T., Dimitrova, D. G., Dinges, N., Lence, T., Worpenberg, L., Carre, C., & Roignant, J. Y. (2018). The emerging field of Epitranscriptomics in neurodevelopmental and neuronal disorders. Frontiers in Bioengineering and Biotechnology, 6, 46.
Gilbert, W. V., Bell, T. A., & Schaening, C. (2016). Messenger RNA modifications: Form, distribution, and function. Science, 352(6292), 1408–1412.
Fustin, J. M., Doi, M., Yamaguchi, Y., Hida, H., Nishimura, S., Yoshida, M., Isagawa, T., Morioka, M. S., Kakeya, H., & Manabe, I. (2013). RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell, 155(4), 793–806.
Wang, X., Zhao, B. S., Roundtree, I. A., Lu, Z., Han, D., Ma, H., Weng, X., Chen, K., Shi, H., & He, C. (2015). N6-methyladenosine modulates messenger RNA translation efficiency. Cell, 161(6), 1388–1399.
Hubstenberger, A., Courel, M., Bénard, M., Souquere, S., Ernoult-Lange, M., Chouaib, R., Yi, Z., Morlot, J. B., Munier, A., & Fradet, M. (2017). P-body purification reveals the condensation of repressed mRNA regulons. Molecular Cell, 68(1), 144–157.
Yang, Y., Hsu, P. J., Chen, Y. S., & Yang, Y. G. (2018). Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Research, 28(6), 616–624.
Bokar, J. A., Shambaugh, M. E., Polayes, D., Matera, A. G., & Rottman, F. M. (1997). Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA, 3(11), 1233–1247.
Zhao, B. S., Wang, X., Beadell, A. V., Lu, Z., Shi, H., Kuuspalu, A., Ho, R. K., & He, C. (2017). M(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature, 542(7642), 475–478.
Chang, M., Lv, H., Zhang, W., Ma, C., He, X., Zhao, S., Zhang, Z. W., Zeng, Y. X., Song, S., Niu, Y., & Tong, W. M. (2017). Region-specific RNA m(6)a methylation represents a new layer of control in the gene regulatory network in the mouse brain. Open Biology, 7(9), 170166.
Li, M., Zhao, X., Wang, W., Shi, H., Pan, Q., Lu, Z., Perez, S. P., Suganthan, R., He, C., Bjoras, M., & Klungland, A. (2018). Ythdf2-mediated m(6)a mRNA clearance modulates neural development in mice. Genome Biology, 19(1), 69.
Yoon, K. J., Ringeling, F. R., Vissers, C., Jacob, F., Pokrass, M., Jimenez-Cyrus, D., Su, Y., Kim, N. S., Zhu, Y., Zheng, L., Kim, S., Wang, X., Dore, L. C., Jin, P., Regot, S., Zhuang, X., Canzar, S., He, C., Ming, G. L., & Song, H. (2017). Temporal control of mammalian cortical neurogenesis by m(6)a methylation. Cell, 171(4), 877–889.
Ma, C., Chang, M., Lv, H., Zhang, Z. W., Zhang, W., He, X., Wu, G., Zhao, S., Zhang, Y., Wang, D., Teng, X., Liu, C., Li, Q., Klungland, A., Niu, Y., Song, S., & Tong, W. M. (2018). RNA m(6)a methylation participates in regulation of postnatal development of the mouse cerebellum. Genome Biology, 19(1), 68.
Wang, C. X., Cui, G. S., Liu, X., Xu, K., Wang, M., Zhang, X. X., Jiang, L. Y., Li, A., Yang, Y., Lai, W. Y., Sun, B. F., Jiang, G. B., Wang, H. L., Tong, W. M., Li, W., Wang, X. J., Yang, Y. G., & Zhou, Q. (2018). METTL3-mediated m6A modification is required for cerebellar development. PLoS Biology, 16(6), e2004880.
Wang, Y., Li, Y., Yue, M., Wang, J., Kumar, S., Wechsler-Reya, R. J., Zhang, Z., Ogawa, Y., Kellis, M., Duester, G., & Zhao, J. C. (2018). N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nature Neuroscience, 21(2), 195–206.
Li, L., Zang, L., Zhang, F., Chen, J., Shen, H., Shu, L., Liang, F., Feng, C., Chen, D., Tao, H., Xu, T., Li, Z., Kang, Y., Wu, H., Tang, L., Zhang, P., Jin, P., Shu, Q., & Li, X. (2017). Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Human Molecular Genetics, 26(13), 2398–2411.
Kimelman, D. (2006). Mesoderm induction: From caps to chips. Nature Reviews. Genetics, 7(5), 360–372.
Zhang, C., Chen, Y., Sun, B., Wang, L., Yang, Y., Ma, D., Lv, J., Heng, J., Ding, Y., Xue, Y., Lu, X., Xiao, W., Yang, Y. G., & Liu, F. (2017). M(6)a modulates haematopoietic stem and progenitor cell specification. Nature, 549(7671), 273–276.
Vu, L. P., Pickering, B. F., Cheng, Y., Zaccara, S., Nguyen, D., Minuesa, G., Chou, T., Chow, A., Saletore, Y., MacKay, M., Schulman, J., Famulare, C., Patel, M., Klimek, V. M., Garrett-Bakelman, F. E., Melnick, A., Carroll, M., Mason, C. E., Jaffrey, S. R., & Kharas, M. G. (2017). The N(6)-methyladenosine (m(6)a)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nature Medicine, 23(11), 1369–1376.
Kudou, K., Komatsu, T., Nogami, J., Maehara, K., Harada, A., Saeki, H., Oki, E., Maehara, Y., & Ohkawa, Y. (2017). The requirement of Mettl3-promoted MyoD mRNA maintenance in proliferative myoblasts for skeletal muscle differentiation. Open Biology, 7(9), 170119.
Ben-Haim, M. S., Moshitch-Moshkovitz, S., & Rechavi, G. (2015). FTO: Linking m6A demethylation to adipogenesis. Cell Research, 25(1), 3–4.
Zhao, X., Yang, Y., Sun, B. F., Shi, Y., Yang, X., Xiao, W., Hao, Y. J., Ping, X. L., Chen, Y. S., Wang, W. J., Jin, K. X., Wang, X., Huang, C. M., Fu, Y., Ge, X. M., Song, S. H., Jeong, H. S., Yanagisawa, H., Niu, Y., Jia, G. F., Wu, W., Tong, W. M., Okamoto, A., He, C., Rendtlew-Danielsen, J. M., Wang, X. J., & Yang, Y. G. (2014). FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Research, 24(12), 1403–1419.
Kobayashi, M., Ohsugi, M., Sasako, T., Awazawa, M., Umehara, T., Iwane, A., Kobayashi, N., Okazaki, Y., Kubota, N., Suzuki, R., Waki, H., Horiuchi, K., Hamakubo, T., Kodama, T., Aoe, S., Tobe, K., Kadowaki, T., & Ueki, K. (2018). The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in Adipogenesis. Molecular and Cellular Biology, 38(16), e00116–e00118.
Hsu, P. J., Zhu, Y., Ma, H., Guo, Y., Shi, X., Liu, Y., Qi, M., Lu, Z., Shi, H., Wang, J., Cheng, Y., Luo, G., Dai, Q., Liu, M., Guo, X., Sha, J., Shen, B., & He, C. (2017). Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Research, 27(9), 1115–1127.
Kasowitz, S. D., Ma, J., Anderson, S. J., Leu, N. A., Xu, Y., Gregory, B. D., Schultz, R. M., & Wang, P. J. (2018). Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genetics, 14(5), e1007412.
Zheng, G., Dahl, J. A., Niu, Y., Fedorcsak, P., Huang, C. M., Li, C. J., Vagbo, C. B., Shi, Y., Wang, W. L., Song, S. H., Lu, Z., Bosmans, R. P., Dai, Q., Hao, Y. J., Yang, X., Zhao, W. M., Tong, W. M., Wang, X. J., Bogdan, F., Furu, K., Fu, Y., Jia, G., Zhao, X., Liu, J., Krokan, H. E., Klungland, A., Yang, Y. G., & He, C. (2013). ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Molecular Cell, 49(1), 18–29.
Lin, Z., Hsu, P. J., Xing, X., Fang, J., Lu, Z., Zou, Q., Zhang, K. J., Zhang, X., Zhou, Y., Zhang, T., Zhang, Y., Song, W., Jia, G., Yang, X., He, C., & Tong, M. H. (2017). Mettl3−/Mettl14-mediated mRNA N(6)-methyladenosine modulates murine spermatogenesis. Cell Research, 27(10), 1216–1230.
Geula, S., Moshitch-Moshkovitz, S., Dominissini, D., Mansour, A. A., Kol, N., Salmon-Divon, M., Hershkovitz, V., Peer, E., Mor, N., Manor, Y. S., Ben-Haim, M. S., Eyal, E., Yunger, S., Pinto, Y., Jaitin, D. A., Viukov, S., Rais, Y., Krupalnik, V., Chomsky, E., Zerbib, M., Maza, I., Rechavi, Y., Massarwa, R., Hanna, S., Amit, I., Levanon, E. Y., Amariglio, N., Stern-Ginossar, N., Novershtern, N., Rechavi, G., & Hanna, J. H. (2015). Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science, 347(6225), 1002–1006.
Lin, S., & Gregory, R. I. (2014). Methyltransferases modulate RNA stability in embryonic stem cells. Nature Cell Biology, 16(2), 129–131.
Wang, Y., Li, Y., Toth, J. I., Petroski, M. D., Zhang, Z., & Zhao, J. C. (2014). N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature Cell Biology, 16(2), 191–198.
Aguilo, F., Zhang, F., Sancho, A., Fidalgo, M., Di Cecilia, S., Vashisht, A., Lee, D. F., Chen, C. H., Rengasamy, M., Andino, B., Jahouh, F., Roman, A., Krig, S. R., Wang, R., Zhang, W., Wohlschlegel, J. A., Wang, J., & Walsh, M. J. (2015). Coordination of m(6)a mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell, 17(6), 689–704.
Chen, T., Hao, Y. J., Zhang, Y., Li, M. M., Wang, M., Han, W., Wu, Y., Lv, Y., Hao, J., Wang, L., Li, A., Yang, Y., Jin, K. X., Zhao, X., Li, Y., Ping, X. L., Lai, W. Y., Wu, L. G., Jiang, G., Wang, H. L., Sang, L., Wang, X. J., Yang, Y. G., & Zhou, Q. (2015). M(6)a RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell, 16(3), 289–301.
Wen, J., Lv, R., Ma, H., Shen, H., He, C., Wang, J., Jiao, F., Liu, H., Yang, P., Tan, L., Lan, F., Shi, Y. G., He, C., Shi, Y., & Diao, J. (2018). Zc3h13 regulates nuclear RNA m(6)a methylation and mouse embryonic stem cell self-renewal. Molecular Cell, 69(6), 1028–1038.
Bertero, A., Brown, S., Madrigal, P., Osnato, A., Ortmann, D., Yiangou, L., Kadiwala, J., Hubner, N. C., de Los Mozos, I. R., Sadee, C., Lenaerts, A. S., Nakanoh, S., Grandy, R., Farnell, E., Ule, J., Stunnenberg, H. G., Mendjan, S., & Vallier, L. (2018). The SMAD2/3 interactome reveals that TGFbeta controls m(6)a mRNA methylation in pluripotency. Nature, 555(7695), 256–259.
Verma, M. K., & Lenka, N. (2010). Temporal and contextual orchestration of cardiac fate by WNT-BMP synergy and threshold. Journal of Cellular and Molecular Medicine, 14(8), 2094–2108.
Faulds, K. J., Egelston, J. N., Sedivy, L. J., Mitchell, M. K., Garimella, S., Kozlowski, H., D'Alessandro, A., Hansen, K. C., Balsbaugh, J. L., & Phiel, C. J. (2018). Glycogen synthase kinase-3 (Gsk-3) activity regulates mRNA methylation in mouse embryonic stem cells. The Journal of Biological Chemistry, 293(27), 10731–10743.
Yang, D., Qiao, J., Wang, G., Lan, Y., Li, G., Guo, X., Xi, J., Ye, D., Zhu, S., Chen, W., Jia, W., Leng, Y., Wan, X., & Kang, J. (2018). N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Research, 46(8), 3906–3920.
Desrosiers, R., Friderici, K., & Rottman, F. (1974). Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proceedings of the National Academy of Sciences, 71(10), 3971–3975.
Squires, J. E., Patel, H. R., Nousch, M., Sibbritt, T., Humphreys, D. T., Parker, B. J., Suter, C. M., & Preiss, T. (2012). Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Research, 40(11), 5023–5033.
Khoddami, V., & Cairns, B. R. (2013). Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nature Biotechnology, 31(5), 458–464.
Yang, X., Yang, Y., Sun, B. F., Chen, Y. S., Xu, J. W., Lai, W. Y., Li, A., Wang, X., Bhattarai, D. P., Xiao, W., Sun, H. Y., Zhu, Q., Ma, H. L., Adhikari, S., Sun, M., Hao, Y. J., Zhang, B., Huang, C. M., Huang, N., Jiang, G. B., Zhao, Y. L., Wang, H. L., Sun, Y. P., & Yang, Y. G. (2017). 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Research, 27(5), 606–625.
Fu, L., Guerrero, C. R., Zhong, N., Amato, N. J., Liu, Y., Liu, S., Cai, Q., Ji, D., Jin, S. G., Niedernhofer, L. J., Pfeifer, G. P., Xu, G. L., & Wang, Y. (2014). Tet-mediated formation of 5-hydroxymethylcytosine in RNA. Journal of the American Chemical Society, 136(33), 11582–11585.
Delatte, B., Wang, F., Ngoc, L. V., Collignon, E., Bonvin, E., Deplus, R., Calonne, E., Hassabi, B., Putmans, P., Awe, S., Wetzel, C., Kreher, J., Soin, R., Creppe, C., Limbach, P. A., Gueydan, C., Kruys, V., Brehm, A., Minakhina, S., Defrance, M., Steward, R., & Fuks, F. (2016). RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science, 351(6270), 282–285.
Guallar, D., Bi, X., Pardavila, J. A., Huang, X., Saenz, C., Shi, X., Zhou, H., Faiola, F., Ding, J., Haruehanroengra, P., Yang, F., Li, D., Sanchez-Priego, C., Saunders, A., Pan, F., Valdes, V. J., Kelley, K., Blanco, M. G., Chen, L., Wang, H., Sheng, J., Xu, M., Fidalgo, M., Shen, X., & Wang, J. (2018). RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nature Genetics, 50(3), 443–451.
Trixl, L., Amort, T., Wille, A., Zinni, M., Ebner, S., Hechenberger, C., Eichin, F., Gabriel, H., Schoberleitner, I., Huang, A., Piatti, P., Nat, R., Troppmair, J., & Lusser, A. (2018). RNA cytosine methyltransferase Nsun3 regulates embryonic stem cell differentiation by promoting mitochondrial activity. Cellular and Molecular Life Sciences, 75(8), 1483–1497.
Amort, T., Rieder, D., Wille, A., Khokhlova-Cubberley, D., Riml, C., Trixl, L., Jia, X. Y., Micura, R., & Lusser, A. (2017). Distinct 5-methylcytosine profiles in poly(a) RNA from mouse embryonic stem cells and brain. Genome Biology, 18(1), 1.
Miao, Z., Xin, N., Wei, B., Hua, X., Zhang, G., Leng, C., Zhao, C., Wu, D., Li, J., Ge, W., Sun, M., & Xu, X. (2016). 5-hydroxymethylcytosine is detected in RNA from mouse brain tissues. Brain Research, 1642, 546–552.
Flores, J. V., Cordero-Espinoza, L., Oeztuerk-Winder, F., Andersson-Rolf, A., Selmi, T., Blanco, S., Tailor, J., Dietmann, S., & Frye, M. (2017). Cytosine-5 RNA methylation regulates neural stem cell differentiation and motility. Stem Cell Reports, 8(1), 112–124.
Blanco, S., Dietmann, S., Flores, J. V., Hussain, S., Kutter, C., Humphreys, P., Lukk, M., Lombard, P., Treps, L., Popis, M., Kellner, S., Holter, S. M., Garrett, L., Wurst, W., Becker, L., Klopstock, T., Fuchs, H., Gailus-Durner, V., Hrabe de Angelis, M., Karadottir, R. T., Helm, M., Ule, J., Gleeson, J. G., Odom, D. T., & Frye, M. (2014). Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. The EMBO Journal, 33(18), 2020–2039.
Cohn, W. E. (1960). Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: Isolation, structure, and chemical characteristics. The Journal of Biological Chemistry, 235, 1488–1498.
Li, X., Zhu, P., Ma, S., Song, J., Bai, J., Sun, F., & Yi, C. (2015). Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nature Chemical Biology, 11(8), 592–597.
Schwartz, S., Bernstein, D. A., Mumbach, M. R., Jovanovic, M., Herbst, R. H., Leon-Ricardo, B. X., Engreitz, J. M., Guttman, M., Satija, R., Lander, E. S., Fink, G., & Regev, A. (2014). Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell, 159(1), 148–162.
Carlile, T. M., Rojas-Duran, M. F., Zinshteyn, B., Shin, H., Bartoli, K. M., & Gilbert, W. V. (2014). Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature, 515(7525), 143–146.
Guzzi, N., Ciesla, M., Ngoc, P. C. T., Lang, S., Arora, S., Dimitriou, M., Pimkova, K., Sommarin, M. N. E., Munita, R., Lubas, M., Lim, Y., Okuyama, K., Soneji, S., Karlsson, G., Hansson, J., Jonsson, G., Lund, A. H., Sigvardsson, M., Hellstrom-Lindberg, E., Hsieh, A. C., & Bellodi, C. (2018). Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell, 173(5), 1204–1216.
Acknowledgements
The work was supported by intramural funding from NCCS to NL and VH is a graduate student supported by fellowship from Council of Scientific and Industrial Research (CSIR), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haran, V., Lenka, N. Deciphering the Epitranscriptomic Signatures in Cell Fate Determination and Development. Stem Cell Rev and Rep 15, 474–496 (2019). https://doi.org/10.1007/s12015-019-09894-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09894-3