Abstract
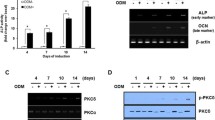
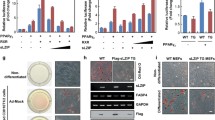
The ionotropic P2X7 receptor (P2X7R) is involved in bone homeostasis but its role in osteogenesis is controversial. Thus, we investigated the expression of P2X7R and the effects exerted by its modulation in mesenchymal stromal cells from human subcutaneous adipose tissue (S-ASCs), which have potential therapeutic application in bone regenerative medicine. We found that undifferentiated S-ASCs expressed P2X7R and its functional splice variants P2X7AR and P2X7BR. Cell stimulation by P2X7R agonist BzATP (100 μM) neither modified proliferation nor caused membrane pore opening while increasing intracellular Ca2+ levels and migration. The P2X7R antagonist A438079 reversed these effects. However, 25-100 μM BzATP, administered to S-ASCs undergoing osteogenic differentiation, dose-dependently decreased extracellular matrix mineralization and expression of osteogenic transcription factors Runx2, alkaline phosphatase and osteopontin. These effects were not coupled to cell proliferation reduction or to cell death increase, but were associated to decrease in P2X7AR and P2X7BR expression. In contrast, expression of P2X7R, especially P2X7BR isoform, significantly increased during the osteogenic process. Noteworthy, the antagonist A438079, administered alone, at first restrained cell differentiation, enhancing it later. Accordingly, A438079 reversed BzATP effects only in the second phase of S-ASCs osteogenic differentiation. Apyrase, a diphosphohydrolase converting ATP/ADP into AMP, showed a similar behavior. Altogether, findings related to A438079 or apyrase effects suggest an earlier and prevailing pro-osteogenic activity by endogenous ATP and a later one by adenosine derived from endogenous ATP metabolism. Conversely, P2X7R pharmacological stimulation by BzATP, mimicking the effects of high ATP levels occurring during tissue injuries, depressed receptor expression/activity impairing MSC osteogenic differentiation.








Similar content being viewed by others
Abbreviations
- A438079:
-
3-[[5-(2,3-Dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride
- ADO:
-
Adnosine
- ALP:
-
Alkaline phosphatase
- ARS:
-
Alizarin Red S
- BzATP:
-
2′(3′)-O-(4-benzoylbenzoyl)-ATP
- FBS:
-
fetal bovine serum
- MSCs:
-
mesenchymal stromal/stem cells
- NF279:
-
8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt
- OPN:
-
osteopontin
- P2X7R:
-
P2X7 receptors
- P2X1R:
-
P2X1 receptors
- P2X3R:
-
P2X3 receptors
- P2X7AR:
-
P2X7 receptor splice variant A
- P2X7BR:
-
P2X7 receptor splice variant B
- qRT-PCR:
-
quantitative real time polymerase chain reaction
- RUNX2:
-
Runt-related transcription factor 2
- S-ASCs:
-
subcutaneous adipose tissue-derived stromal stem cells.
References
Salem, H. K., & Thiemermann, C. (2010). Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells, 28, 585–596. https://doi.org/10.1002/stem.269.
Egusa, H., Sonoyama, W., Nishimura, M., Atsuta, I., & Akiyama, K. (2012). Stem cells in dentistry–part I: Clinical applications. Journal of Prosthodontic Research, 56, 229–248. https://doi.org/10.1016/j.jpor.2012.10.001.
Wagner, W., Horn, P., Castoldi, M., Diehlmann, A., Bork, S., Saffrich, R., Benes, V., Blake, J., Pfister, S., Eckstein, V., & Ho, A. D. (2008). Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS One, 3, e2213.
Li, S., Wang, Y., Guan, L., & Ji, M. (2015). Characteristics of human umbilical cord mesenchymal stem cells during ex vivo expansion. Molecular Medicine Reports, 12, 4320–43255. https://doi.org/10.3892/mmr.2015.3999.
Dufrane, D. (2017). Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplantation, 26, 1496–1504. https://doi.org/10.1177/0963689717721203.
Lavoie, J. R., & Rosu-Myles, M. (2013). Uncovering the secretes of mesenchymal stem cells. Biochimie, 95, 2212–2221. https://doi.org/10.1016/j.biochi.2013.06.017.
Lazarowski, E. R. (2012). Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal, 8, 359–373. https://doi.org/10.1007/s11302-012-9304-9.
Noronha-Matos, J. B., & Correia-de-Sá, P. (2016). Mesenchymal stem cells ageing: Targeting the "Purinome" to promote osteogenic differentiation and bone repair. Journal of Cellular Physiology, 231, 1852–1861. https://doi.org/10.1002/jcp.25303.
Fredholm, B. B., IJzerman, A. P., Jacobson, K. A., Linden, J., & Muller, C. E. (2011). International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacological Reviews, 63, 1–34. https://doi.org/10.1124/pr.110.003285.
Burnstock, G. (2014). Purinergic signalling: From discovery to current developments. Experimental Physiology, 99, 16–34. https://doi.org/10.1113/expphysiol.2013.071951.
Gartland, A., Orriss, I. R., Rumney, R. M., Bond, A. P., Arnett, T., & Gallagher, J. A. (2012). Purinergic signalling in osteoblasts. Frontiers in Bioscience, 17, 16–29.
Jorgensen, N. R., Syberg, S., & Ellegaard, M. (2015). The role of P2X receptors in bone biology. Current Medicinal Chemistry, 22, 902–914.
Orriss, I. R., Guneri, D., Hajjawi, M.O.R., Shaw, K., Patel, J. J., &. Arnett, T. R. (2017) Activation of the P2Y(2) receptor regulates bone cell function by enhancing ATP release. The Journal of Endocrinology, 233, 341–356. doi: https://doi.org/10.1530/JOE-17-0042.
Jiang, L.-H., Hao, Y., Mousawi, F., Peng, H., & Yang, X. (2017). Expression of P2 purinergic receptors in mesenchymal stem cells and their roles in extracellular nucleotide regulation of cell functions. Journal of Cellular Physiology, 232, 287–297. https://doi.org/10.1002/jcp.25484.
Gharibi, B., Abraham, A. A., Ham, J., & Evans, B. A. (2011). Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. Journal of Bone and Mineral Research, 26, 2112–2124. https://doi.org/10.1002/jbmr.424.
Carrol, S. H., Wigner, N. A., Kulkarni, N., Johnston-Cox, H., Gerstenfeld, L. C., & Ravid, K. (2012). A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. The Journal of Biological Chemistry, 287, 15718–15727. https://doi.org/10.1074/jbc.M112.344994.
He, W., Mazumder, A., Wilder, T., & Cronstein, B. N. (2013). Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. The FASEB Journal, 27, 3446–3454. https://doi.org/10.1096/fj.13-231233.
Ciciarello, M., Zini, R., Rossi, L., Salvestrini, V., Ferrari, D., Manfredini, R., & Lemoli, R. M. (2013). Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells and Development, 22, 1097–1111. https://doi.org/10.1089/scd.2012.0432.
Agrawal, A., & Gartland, A. (2015). P2X7 receptors: Role in bone cell formation and function. Journal of Molecular Endocrinology, 54, R75–R88. https://doi.org/10.1530/JME-14-0226.
Lenertz, L. Y., Baughman, C. J., Waldschmidt, N. V., Thaler, R., & van Wijnen, A. J. (2015). Control of bone development by P2X and P2Y receptors expressed in mesenchymal and hematopoietic cells. Gene, 570, 1–7. https://doi.org/10.1016/j.gene.2015.06.031.
Jiang, L. H., Baldwin, J. M., Roger, S., & Baldwin, S. A. (2013). Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Frontiers in Pharmacology, 4, 55. https://doi.org/10.3389/fphar.2013.00055.
Sluyter, R. (2017). The P2X7 receptor. Advances in Experimental Medicine and Biology, 1051, 17–53. https://doi.org/10.1007/5584_2017_59.
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., & Buell, G. (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science, 272, 735–738. https://doi.org/10.1126/science.272.522.735.
Cheewatrakoolpong, B., Gilchrest, H., Anthes, J. C., & Greenfeder, S. (2005). Identification and characterization of splice variants of the human P2X7 ATP channel. Biochemical and Biophysical Research Communications, 332, 17–27.
Feng, Y. H., Li, X., Wang, L., Zhou, L., & Gorodeski, G. I. (2006). A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. The Journal of Biological Chemistry, 281, 17228–17237.
Adinolfi, E., Cirillo, M., Woltersdorf, R., Falzoni, S., Chiozzi, P., Pellegatti, P., Callegari, M. G., Sandonà, D., Markwardt, F., Schmalzing, G., & Di Virgilio, F. (2010). Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. The FASEB Journal, 24, 3393–3404. https://doi.org/10.1096/fj.09-153601.
Caprara, G. A., Morabito, C., Perni, S., Navarra, R., Guarnieri, S., & Mariggio, M. A. (2016). Evidence for altered Ca2+ handling in growth associated protein 43-knockout skeletal muscle. Frontiers in Physiology, 7, 493 eCollection 2016.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−11CT method. Methods, 25, 402–408. https://doi.org/10.1006/meth.2001.1262.
D’Alimonte, I., Mastrangelo, F., Giuliani, P., Pierdomenico, L., Marchisio, M., Zuccarini, M., Di Iorio, P., Quaresima, R., Caciagli, F., & Ciccarelli, R. (2017). Osteogenic differentiation of mesenchymal stromal cells: A comparative analysis between human subcutaneous adipose tissue and dental pulp. Stem Cells and Development, 26, 843–855. https://doi.org/10.1089/scd.2016.0190.
Bianchi, B. R., Lynch, K. J., Touma, E., Niforatos, W., Burgard, E. C., Alexander, K. M. H., Park, S., Yu, H., Metzger, R., Kowaluk, E., Jarvis, M. F., & van Biesen, T. (1999). Pharmacological characterization of recombinant human and rat P2X receptor subtypes. European Journal of Pharmacology, 376, 127–138.
Kotova, P. D., Bystrova, M. F., Rogachevskaja, O. A., Khokhlov, A. A., Sysoeva, V. Y., Tkachuk, V. A., & Kolesnikov, S. S. (2018). Coupling of P2Y receptors to ca(2+) mobilization in mesenchymal stromal cells from the human adipose tissue. Cell Calcium, 71, 1–14. https://doi.org/10.1016/j.ceca.2017.11.001.
Giuliani, A. L., Colognesi, D., Ricco, T., Roncato, C., Capece, M., Amoroso, F., Wang, Q. G., De Marchi, E., Gartland, A., Di Virgilio, F., & Adinolfi, E. (2014). Trophic activity of human P2X7 receptor isoforms a and B in osteosarcoma. PLoS One, 9, e107224. https://doi.org/10.1371/journal.pone.0107224.
Forostyak, O., Butenko, O., Anderova, M., Forostyak, S., Sykova, E., Verkhratsky, A., & Dayanithi, G. (2016). Specific profiles of ion channels and ionotropic receptors define adipose- and bone marrow derived stromal cells. Stem Cell Research, 16, 622–634. https://doi.org/10.1016/j.scr.2016.03.010.
Ali, S., Turner, J., & Fountain, S. J. (2018) P2Y(2) and P2Y(6) receptor activation elicits intracellular calcium responses in human adipose-derived mesenchymal stromal cells. Purinergic Signal. Aug. 7 [Epub ahead of print] https://doi.org/10.1007/s11302-018-9618-3.
Xing, S., Grol, M. W., Grutter, P. H., Dixon, S. J., & Komarova, S. V. (2016). Modeling interactions among individual P2 receptors to explain complex response patterns over a wide range of ATP concentrations. Frontiers in Physiology, 7, 294. https://doi.org/10.3389/fphys.2016.00294.
Gartland, A., Hipskind, R. A., Gallagher, J. A., & Bowler, W. B. (2001). Expression of a P2X7 receptor by a subpopulation of human osteoblasts. Journal of Bone and Mineral Research, 16, 846–856.
Nakamura, E., Uezono, Y., Narusawa, K., Shibuya, I., Oishi, Y., Tanaka, M., Yanagihara, N., Nakamura, T., & Izumi, F. (2000). ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. American Journal of Physiology. Cell Physiology, 279, C510–C519.
Tamajusuku, A. S., Villodre, E. S., Paulus, R., Coutinho-Silva, R., Battasstini, A. M., Wink, M. R., & Lenz, G. (2010). Characterization of ATP-induced cell death in the GL261 mouse glioma. Journal of Cellular Biochemistry, 109, 983–991. https://doi.org/10.1002/jcb.22478.
Agrawal, A., Henriksen, Z., Syberg, S., Petersen, S., Aslan, D., Solgaard, M., Nissen, N., Larsen, T. K., Schwarz, P., Steinberg, T. H., & Jørgensen, N. R. (2017). P2X7Rs are involved in cell death, growth and cellular signaling in primary human osteoblasts. Bone., 95, 91–101. https://doi.org/10.1016/j.bone.2016.11.011.
Jiang, L. H., Mousawi, F., Yang, X., & Roger, S. (2017). ATP-induced ca(2+)-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cellular and Molecular Life Sciences, 74, 3697–3710. https://doi.org/10.1007/s00018-017-2545-6.
Adinolfi, E., Capece, M., Franceschini, A., Falzoni, S., Giuliani, A. L., Rotondo, A., Sarti, A. C., Bonora, M., Syberg, S., Corigliano, D., Pinton, P., Jorgensen, N. R., Abelli, L., Emionite, L., Raffaghello, L., Pistoia, V., & Di Virgilio, F. (2015). Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Research, 75, 635–644. https://doi.org/10.1158/0008-5472.CAN-14-1259.
Orriss, I. R., Knight, G. E., Ranasinghe, S., Burnstock, G., & Arnett, T. R. (2006). Osteoblast responses to nucleotides increase during differentiation. Bone, 39, 300–309.
Orriss, I. R., Key, M. L., Brandao-Burch, A., Patel, J. J., Burnstock, G., & Arnett, T. R. (2012). The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of P2X receptors. Bone, 51, 389–400. https://doi.org/10.1016/j.bone.2012.06.013.
Panupinthu, N., Rogers, R. J., Zhao, L., Solano-Flores, L. P., Possmayer, F., Sims, S. M., & Dixon, S. J. (2008). P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: A signaling axis promoting osteogenesis. The Journal of Cell Biology, 181, 859–871. https://doi.org/10.1083/jcb.200708037.
Noronha-Matos, J. B., Coimbra, J., Sa-e-Sousa, R. R., Marinhas, J., Freitas, R., Guerra-Gomes, S., Ferreirinha, F., Costa, M. A., & Correia-de-Sá, P. (2014). P2X7-induced zeiosis promotes osteogenic differentiation andmineralization of postmenopausal bone marrow-derived mesenchymal stem cells. The FASEB Journal, 28, 5208–5222. https://doi.org/10.1096/fj.14-257923.
Gangadharan, V., Nohe, A., Caplan, J., Czymmek, K., & Duncan, R. L. (2015). Caveolin-1 regulates P2X7 receptor signaling in osteoblasts. American Journal of Physiology. Cell Physiology, 308, C41–C50. https://doi.org/10.1152/ajpcell.00037.2014.
Di Virgilio, F., Schmalzing, G., & Markwardt, F. (2018). The elusive P2X7 macropore. Trends in Cell Biology, 28, 392–404. https://doi.org/10.1016/j.tcb.2018.01.005.
Mackenzie, A. B., Young, M. T., Adinolfi, E., & Surprenant, A. (2005). Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. The Journal of Biological Chemistry, 280, 33968–33976.
Miyazaki, T., Iwasawa, M., Nakashima, T., Mori, S., Shigemoto, K., Nakamura, H., Katagiri, H., Takayanagi, H., & Tanaka, S. (2012). Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. The Journal of Biological Chemistry, 287, 37808–37823. https://doi.org/10.1074/jbc.M112.385369.
Funding
This work was supported by funds for research to RC and PDI from the University of Chieti-Pescara.
Author information
Authors and Affiliations
Contributions
FC, PDI and RC conceived and designed the experimental work, assembled and supervised the overall project, analyzed and interpreted the data and finalized the manuscript. MC initiated the project and along with SZ, MZ, PG, CM, ML, MAM, EA and EO performed the experimental work, assembled and analyzed the data, drafted/revised the manuscript. All authors have read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Consent for Publication
This manuscript has been approved by all authors and is solely the work of the authors named.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marzia Carluccio and Mariachiara Zuccarini equally co-authored
Electronic supplementary material
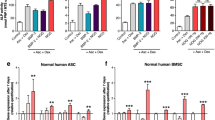
Expression of P2X1R and P2X3R in S-ASCs along their osteogenic differentiation in the presence or absence of BzATP.
Protein levels of P2X1R and P2X3R were determined by Western blot analysis (60 μg of proteins were loaded per lane). Immunoblots were obtained by exposing membranes to two antibodies, recognizing proteins (the monomeric subunit of each receptor) at 50 KDa (A) and about 60 KDa (B), respectively. Subsequently, immunoblots were reprobed with an antibody against β actin, to verify equal sample loading, and quantified by densitometric analysis, the values of which, normalized to β actin, are reported as such in the two lower histograms, whereas in the upper histograms they are reported assuming the values of cells at the day 0 equal to 1. All values are the mean ± SEM of three independent experiments with very similar results. *P < 0.05, **P < 0.01, ***P < 0.001: statistical significance vs. S-ASCs at the day 0 (in the upper histograms) or vs. cells grown in osteogenic medium for 7 days (in the lower histograms); #P < 0.05, ###P < 0.001: statistical significance of values measured in cells exposed to BzATP vs the corresponding untreated cells grown in osteogenic medium (one-way ANOVA plus Dunnett’s test). (PPTX 326 kb)
Rights and permissions
About this article
Cite this article
Carluccio, M., Zuccarini, M., Ziberi, S. et al. Involvement of P2X7 Receptors in the Osteogenic Differentiation of Mesenchymal Stromal/Stem Cells Derived from Human Subcutaneous Adipose Tissue. Stem Cell Rev and Rep 15, 574–589 (2019). https://doi.org/10.1007/s12015-019-09883-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09883-6