Abstract
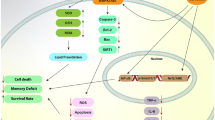
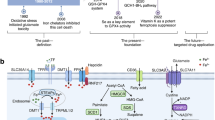
Ferroptosis is a recently identified form of cell death characterized by iron accumulation and lipid peroxidation. Unlike apoptosis, necrosis, and autophagy, ferroptosis operates through a distinct molecular pathway. Curcumin, derived from turmeric rhizomes, is a natural compound with diverse therapeutic benefits, including neuroprotective, anti-metabolic syndrome, anti-inflammatory, and anti-cancer properties. Growing evidence suggests that curcumin possesses both pro-oxidant and antioxidant properties, which can vary depending on the cell type. In this review, we explore the relationship between the effects of curcumin and the molecular mechanisms underlying the ferroptosis signaling pathway, drawing from current in vivo and in vitro research. Curcumin has been found to induce ferroptosis in cancer cells while acting as an inhibitor of ferroptosis in tissue injuries. Notably, curcumin treatment leads to alterations in key ferroptosis markers, underscoring its significant impact on this process. Nonetheless, further research focused on elucidating this important attribute of turmeric is crucial for advancing disease treatment.

Similar content being viewed by others
References
Galluzzi, L., Vitale, I. & Aaronson, S. A. et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death & Differentiation, 25(3), 486–541. https://doi.org/10.1038/s41418-017-0012-4.
Yang, W. S., & Stockwell, B. R. (2016). Ferroptosis: death by lipid peroxidation. Trends in Cell Biology, 26(3), 165–176. https://doi.org/10.1016/j.tcb.2015.10.014.
Xie, L.-H., Fefelova, N., Pamarthi, S. H., & Gwathmey, J. K. (2022). Molecular mechanisms of ferroptosis and relevance to cardiovascular disease. Cells, 11(17), 2726 https://doi.org/10.3390/cells11172726.
Dixon, S. J., Lemberg, K. M., & Lamprecht, M. R., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072. https://doi.org/10.1016/j.cell.2012.03.042.
Yang, W. S., Kim, K. J., Gaschler, M. M., Patel, M., Shchepinov, M. S., & Stockwell, B. R. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proceedings of the National Academy of Sciences USA, 113(34), E4966–E4975. https://doi.org/10.1073/pnas.1603244113.
Forcina, G. C., & Dixon, S. J. (2019). GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics, 19(18), 1800311 https://doi.org/10.1002/pmic.201800311.
Koppula, P., Zhuang, L., & Gan, B. (2021). Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein & Cell, 12(8), 599–620. https://doi.org/10.1007/s13238-020-00789-5.
Prasad, S., Tyagi, A. K., & Aggarwal, B. B. (2014). Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Research and Treatment, 46(1), 2–18. https://doi.org/10.4143/crt.2014.46.1.2.
Cicero, A. F. G., Sahebkar, A., Fogacci, F., Bove, M., Giovannini, M., & Borghi, C. (2020). Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. European Journal of Nutrition, 59(2), 477–483. https://doi.org/10.1007/s00394-019-01916-7.
Keihanian, F., Saeidinia, A., Bagheri, R. K., Johnston, T. P., & Sahebkar, A. (2018). Curcumin, hemostasis, thrombosis, and coagulation. Journal of Cellular Physiology, 233(6), 4497–4511. https://doi.org/10.1002/jcp.26249.
Khayatan D., Razavi S. M., Arab Z. N., et al. Protective effects of curcumin against traumatic brain injury. Biomedicine and Pharmacotherapy. 2022;154. https://doi.org/10.1016/j.biopha.2022.113621
Marjaneh, R. M., Rahmani, F., & Hassanian, S. M., et al. (2018). Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. Journal of Cellular Physiology, 233(10), 6785–6798. https://doi.org/10.1002/jcp.26538.
Mohajeri, M., & Sahebkar, A. (2018). Protective effects of curcumin against doxorubicin-induced toxicity and resistance: a review. Critical Reviews in Oncology/Hematology, 122, 30–51. https://doi.org/10.1016/j.critrevonc.2017.12.005.
Mohammadi, A., Blesso, C. N., Barreto, G. E., Banach, M., Majeed, M., & Sahebkar, A. (2019). Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. Journal of Nutritional Biochemistry, 66, 1–16. https://doi.org/10.1016/j.jnutbio.2018.12.005.
Mokhtari-Zaer, A., Marefati, N., Atkin, S. L., Butler, A. E., & Sahebkar, A. (2018). The protective role of curcumin in myocardial ischemia–reperfusion injury. Journal of Cellular Physiology, 234(1), 214–222. https://doi.org/10.1002/jcp.26848.
Panahi, Y., Fazlolahzadeh, O., & Atkin, S. L., et al. (2019). Evidence of curcumin and curcumin analogue effects in skin diseases: a narrative review. Journal of Cellular Physiology, 234(2), 1165–1178. https://doi.org/10.1002/jcp.27096.
Panahi, Y., Sahebkar, A., & Amiri, M., et al. (2012). Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: Results of a randomised, double-blind, placebo-controlled trial. British Journal of Nutrition, 108(7), 1272–1279. https://doi.org/10.1017/S0007114511006544.
Sahebkar A. Curcuminoids for the management of hypertriglyceridaemia. Nature Reviews Cardiology. 2014;11(2). https://doi.org/10.1038/nrcardio.2013.140-c1
Fu, Y.-S., Chen, T.-H., Weng, L., Huang, L., Lai, D., & Weng, C.-F. (2021). Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomedicine & Pharmacotherapy, 141, 111888 https://doi.org/10.1016/j.biopha.2021.111888.
Grynkiewicz G., Ślifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochimica Polonica. 2012;59(2). https://doi.org/10.18388/abp.2012_2139
Kunwar, A., Sandur, S. K., Krishna, M., & Priyadarsini, K. I. (2009). Curcumin mediates time and concentration dependent regulation of redox homeostasis leading to cytotoxicity in macrophage cells. European Journal of Pharmacology, 611(1-3), 8–16. https://doi.org/10.1016/j.ejphar.2009.03.060.
AloK, A., Singh, I. D., Singh, S., Kishore, M., & Jha, P. C. (2015). Curcumin–pharmacological actions and its role in oral submucous fibrosis: a review. Journal of Clinical and Diagnostic Research, 9(10), ZE01 https://doi.org/10.7860/JCDR/2015/13857.6552.
Ahmed, S., Khan, H., & Mirzaei, H. (2019). Mechanics insights of curcumin in myocardial ischemia: Where are we standing? European Journal of Medicinal Chemistry, 183, 111658 https://doi.org/10.1016/j.ejmech.2019.111658.
Bernfeld, E., & Foster, D. A. (2019). Glutamine as an essential amino acid for KRas-driven cancer cells. Trends in Endocrinology & Metabolism, 30(6), 357–368. https://doi.org/10.1016/j.tem.2019.03.003.
Yang, J., Dai, X., Xu, H., Tang, Q., & Bi, F. (2022). Regulation of ferroptosis by amino acid metabolism in cancer. International Journal of Biological Sciences, 18(4), 1695 https://doi.org/10.7150/ijbs.64982.
Cao, X., Li, Y., & Wang, Y., et al. (2022). Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS ONE, 17(1), e0261370 https://doi.org/10.1371/journal.pone.0261370.
Li R., Zhang J., Zhou Y., et al. Transcriptome investigation and in vitro verification of curcumin-induced HO-1 as a feature of ferroptosis in breast cancer cells. Oxidative Medicine and Cellular Longevity. 2020;2020. https://doi.org/10.1155/2020/3469840
Kwon, M.-Y., Park, E., Lee, S.-J., & Chung, S. W. (2015). Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget, 6(27), 24393 https://doi.org/10.18632/oncotarget.5162.
Chen, T.-C., Chuang, J.-Y., & Ko, C.-Y., et al. (2020). AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biology, 30, 101413 https://doi.org/10.1016/j.redox.2019.101413.
Pignanelli, C., Ma, D., & Noel, M., et al. (2017). Selective targeting of cancer cells by oxidative vulnerabilities with novel curcumin analogs. Scientific Reports, 7(1), 1105 https://doi.org/10.1038/s41598-017-01230-4.
Chen M., Tan A-h, Li J. Curcumin represses colorectal cancer cell proliferation by triggering ferroptosis via PI3K/Akt/mTOR signaling. Nutrition and Cancer. 2022:1–8. https://doi.org/10.1080/01635581.2022.2139398
Zheng, Z., Zhang, X., Carbo, D., Clark, C., Nathan, C.-A., & Lvov, Y. (2010). Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir, 26(11), 7679–7681. https://doi.org/10.1021/la101246a.
Wang, Q., Dou, Y., & Zhao, S., et al. (2020). Analysis of chemical consistency and the anti-tumor activity of Huangqi-Ezhu (HQ-EZ) concentrated-granules and decoction. Ann Palliat Med, 9(4), 1648–1659. https://doi.org/10.21037/apm-19-592.
Zhang, R., Pan, T., & Xiang, Y., et al. (2022). Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioactive Materials, 13, 23–36. https://doi.org/10.1016/j.bioactmat.2021.11.013.
Wang W., Xie Y., Malhotra A. Potential of curcumin and quercetin in modulation of premature mitochondrial senescence and related changes during lung carcinogenesis. Journal of Environmental Pathology Toxicology and Oncology. 2021;40(4). https://doi.org/10.1615/jenvironpatholtoxicoloncol.2021039371
Tang, X., Ding, H., & Liang, M., et al. (2021). Curcumin induces ferroptosis in non‐small‐cell lung cancer via activating autophagy. Thoracic Cancer, 12(8), 1219–1230. https://doi.org/10.1111/1759-7714.13904.
Doll, S., Proneth, B., & Tyurina, Y. Y., et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology, 13(1), 91–98. https://doi.org/10.1038/nchembio.2239.
Kose, T., Vera-Aviles, M., Sharp, P. A., & Latunde-Dada, G. O. (2019). Curcumin and (−)-epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals, 12(1), 26 https://doi.org/10.3390/ph12010026.
Dodson, M., Castro-Portuguez, R., & Zhang, D. D. (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biology, 23, 101107 https://doi.org/10.1016/j.redox.2019.101107.
Wei Z., Shaohuan Q., Pinfang K., Chao S. Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovascular Therapeutics. 2022;2022. https://doi.org/10.1155/2022/3159717
Ursini, F., & Maiorino, M. (2020). Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radical Biology and Medicine, 152, 175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027.
Ikawa, T., Sato, M., Oh-Hashi, K., Furuta, K., & Hirata, Y. (2021). Oxindole–curcumin hybrid compound enhances the transcription of γ-glutamylcysteine ligase. European Journal of Pharmacology, 896, 173898 https://doi.org/10.1016/j.ejphar.2021.173898.
Hirata, Y., Tsunekawa, Y., & Takahashi, M., et al. (2021). Identification of novel neuroprotective N, N-dimethylaniline derivatives that prevent oxytosis/ferroptosis and localize to late endosomes and lysosomes. Free Radical Biology and Medicine, 174, 225–235. https://doi.org/10.1016/j.freeradbiomed.2021.08.015.
Hirata, Y., Okazaki, R., Sato, M., Oh-Hashi, K., Takemori, H., & Furuta, K. (2022). Effect of ferroptosis inhibitors oxindole-curcumin hybrid compound and N, N-dimethylaniline derivatives on rotenone-induced oxidative stress. European Journal of Pharmacology, 928, 175119 https://doi.org/10.1016/j.ejphar.2022.175119.
Zhou, S.-Y., Cui, G.-Z., & Yan, X.-L., et al. (2020). Mechanism of ferroptosis and its relationships with other types of programmed cell death: insights for potential interventions after intracerebral hemorrhage. Frontiers in Neuroscience, 14, 589042 https://doi.org/10.3389/fnins.2020.589042.
Yang, C., Han, M., & Li, R., et al. (2021). Curcumin nanoparticles inhibiting ferroptosis for the enhanced treatment of intracerebral hemorrhage. International Journal of Nanomedicine, 16, 8049 https://doi.org/10.2147/IJN.S334965.
Zheng, Y., Zhao, T., & Wang, J., et al. (2022). Curcumol alleviates liver fibrosis through inducing autophagy and ferroptosis in hepatic stellate cells. The FASEB Journal, 36(12), e22665 https://doi.org/10.1096/fj.202200933RR.
Tang, X., Li, Z., & Yu, Z., et al. (2021). Effect of curcumin on lung epithelial injury and ferroptosis induced by cigarette smoke. Human & Experimental Toxicology, 40(12_suppl), S753–S762. https://doi.org/10.1177/09603271211059497.
Córdoba-David, G., Duro-Castano, A., & Castelo-Branco, R. C., et al. (2020). Effective nephroprotection against acute kidney injury with a star-shaped polyglutamate-curcuminoid conjugate. Scientific Reports, 10(1), 1–15. https://doi.org/10.1038/s41598-020-58974-9.
Guerrero‐Hue, M., García‐Caballero, C., & Palomino‐Antolín, A., et al. (2019). Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis‐mediated cell death. The FASEB Journal, 33(8), 8961–8975. https://doi.org/10.1096/fj.201900077R.
Author information
Authors and Affiliations
Contributions
Z.F. and G.K.- researched the literature and wrote the first draft of the manuscript. A.S.- conceptualization and editing. A.E.B. edited the manuscript. All authors approved the final version of the manuscript. A.S. is the guarantor of this work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Consent to Publication
All authors gave their consent for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Foroutan, Z., Butler, A.E., Zengin, G. et al. Curcumin and Ferroptosis: a Promising Target for Disease Prevention and Treatment. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-023-01212-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-023-01212-6