Abstract
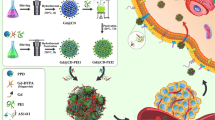
Aptamer-Carbon Dot (CD) bioconjugation is an attractive target-tracking strategy in detecting cell surface antigens. This study describes an effective imaging paradigm for CA-125 antigen imaging. Our experience encompasses green CD synthesis and characterization, CD-capture probe conjugation through covalent bonding, the hybridization linkage of CD-probe to aptamer and their coupling confirmation, and fluorescent targeted imaging of ovarian cancer cells. As a result, the synthesized CDs from lemon extract by hydrothermal reaction show average size of 2 nm with maximum fluorescence intensity at excitation/emission 360/450 nm. CD-probe construction was provided by functional group interactions of CD and probe via EDC/NHS chemistry. The linkage of CD-probe to aptamer was conducted by Watson–Crick nucleotide pairing. The assessment of CD-probe and CD-probe-aptamer fabrication was validated by the increase in surface roughness through AFM analysis, the diminish of fluorescence intensity of CD after bioconjugation, and particle size growth of the construct. Conjugates with negligible cytotoxicity, appropriate zeta potential, and good aptamer release were applied in cellular imaging. This targeted diagnosis method was employed the four reported DNA aptamers toward fluorescence intensity. The DOV-3 aptamer showed more qualified detection over other aptamer conjugates during fluorescent microscopy analysis. In conclusion, the CD-probe-aptamer conjugate applications as toxic-free method can open new horizons in fluorescent nano-imaging in the field of targeted cancer cell diagnosis.









Similar content being viewed by others
References
Faramarzi, L., Dadashpour, M., Sadeghzadeh, H., Mahdavi, M., & Zarghami, N. (2019). Enhanced anti-proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artificial Cells, Nanomedicine and Biotechnology, 47(1), 737–746. https://doi.org/10.1080/21691401.2019.1573737.
Jayson, G. C., Kohn, E. C., Kitchener, H. C., & Ledermann, J. A. (2014). Ovarian cancer. The Lancet, 384(9951), 1376–1388.
Goff, B. A., Mandel, L. S., Drescher, C. W., Urban, N., Gough, S., Schurman, K. M., & Andersen, M. R. (2007). Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer, 109(2), 221–227. https://doi.org/10.1002/cncr.22371.
Akbarzadeh, M., Majidinia, M., Aval, S. F., Mahbub, S., & Zarghami, N. (2018). Molecular targeting of notch signaling pathway by DAPT in human ovarian cancer: possible anti metastatic effects. Asian Pacific journal of cancer prevention: APJCP, 19(12), 3473 https://doi.org/10.31557/APJCP.2018.19.12.3473.
Yilmaz, E. Z., & Yoruker, E. E. (2020). Predictive and Prognostic Markers for Cancer Medicine. Precision Medicine in Oncology. https://doi.org/10.1002/9781119432487.ch6.
Nimse, S. B., Sonawane, M. D., Song, K.-S., & Kim, T. (2016). Biomarker detection technologies and future directions. Analyst, 141(3), 740–755. https://doi.org/10.1039/C5AN01790D.
Chen, A., & Yang, S. (2015). Author’ s Accepted Manuscript Replacing Antibodies With Aptamers In Lateral Flow Immunoassay Reference: Biosensors and bioelectronics, 71, 230–242. https://doi.org/10.1016/j.bios.2015.04.041.
Sun, H., & Zu, Y. (2015). A Highlight of recent advances in aptamer technology and its application. Molecules. Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/molecules200711959.
Marcus, C. S., Maxwell, G. L., Darcy, K. M., Hamilton, C. A., & McGuire, W. P. (2014). Current approaches and challenges in managing and monitoring treatment response in ovarian cancer. Journal of Cancer, 5(1), 25 https://doi.org/10.7150/jca.7810.
Charkhchi, P., Cybulski, C., Gronwald, J., Wong, F. O., Narod, S. A., & Akbari, M. R. (2020). Ca125 and ovarian cancer: a comprehensive review. Cancers, 12(12), 1–29. https://doi.org/10.3390/cancers12123730.
Felder, M., Kapur, A., Gonzalez-Bosquet, J., Horibata, S., Heintz, J., Albrecht, R., & Patankar, M. S. (2014). MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Molecular Cancer, 13(1), 129 https://doi.org/10.1186/1476-4598-13-129.
Rao, T. D., Park, K. J., Smith-Jones, P., Iasonos, A., Linkov, I., Soslow, R. A., & Spriggs, D. R. (2010). Novel monoclonal antibodies against the proximal (carboxy-terminal) portions of MUC16. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry, 18(5), 462 https://doi.org/10.1097/PAI.0b013e3181dbfcd2.Novel.
Kudłak, B., & Wieczerzak, M. (2020). Aptamer based tools for environmental and therapeutic monitoring: a review of developments, applications, future perspectives. Critical Reviews in Environmental Science and Technology, 50(8), 816–867. https://doi.org/10.1080/10643389.2019.1634457.
Jo, H., & Ban, C. (2016). Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Experimental and Molecular Medicine, 48(5), e230 https://doi.org/10.1038/emm.2016.44.
Matthew, R. D., R., M. J., & J., C. C. (2017). Analysis of aptamer discovery and technology. Nature Reviews Chemistry, 1(April), 2720 https://doi.org/10.1038/s41570017-0076.
Pang, X., Cui, C., Wan, S., Jiang, Y., Zhang, L., Xia, L., & Tan, W. (2018). Bioapplications of cell-SELEX-generated aptamers in cancer diagnostics, therapeutics, theranostics and biomarker discovery: a comprehensive review. Cancers, 10(2), 47 https://doi.org/10.3390/cancers10020047.
Shangguan, D., Cao, Z., Meng, L., Mallikaratchy, P., Sefah, K., Wang, H., & Tan, W. (2008). Cell-specific aptamer probes for membrane protein elucidation in cancer cells. Journal of Proteome Research, 7(5), 2133–2139.
Yoon, S., & Rossi, J. J. (2018). Targeted molecular imaging using aptamers in cancer. Pharmaceuticals, 11(3), 71 https://doi.org/10.3390/ph11030071.
Santos-Cancel, M., Simpson, L. W., Leach, J. B., & White, R. J. (2019). Direct, real-time detection of adenosine triphosphate release from astrocytes in three-dimensional culture using an integrated electrochemical aptamer-based sensor. ACS Chemical Neuroscience, 10(4), 2070–2079. https://doi.org/10.1021/acschemneuro.9b00033. research-article.
Mohajeri, N., Imani, M., Akbarzadeh, A., Sadighi, A., & Zarghami, N. (2019). An update on advances in new developing DNA conjugation diagnostics and ultra-resolution imaging technologies: possible applications in medical and biotechnological utilities. Biosensors and Bioelectronics, 144(June), 111633 https://doi.org/10.1016/j.bios.2019.111633.
Li, C. H., Kuo, T. R., Su, H. J., Lai, W. Y., Yang, P. C., Chen, J. S., & Chen, C. C. (2015). Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Scientific Reports, 5(Sept), 15675 https://doi.org/10.1038/srep15675.
Kim, M. W., Jeong, H. Y., Kang, S. J., Choi, M. J., You, Y. M., Im, C. S., & Rhee, K.-J. (2017). Cancer-targeted nucleic acid delivery and quantum dot imaging using EGF receptor aptamer-conjugated lipid nanoparticles. Scientific Reports, 7(1), 9474 https://doi.org/10.1038/s41598-017-09555-w.
Guo, Y., Zhao, C., Liu, Y., Nie, H., Guo, X., Song, X., & Wang, J. (2020). A novel fluorescence method for the rapid and effective detection of: Listeria monocytogenes using aptamer-conjugated magnetic nanoparticles and aggregation-induced emission dots. Analyst, 145(11), 3857–3863. https://doi.org/10.1039/d0an00397b.
Kim, B., Yang, J., Hwang, M., Choi, J., Kim, H.-O., Jang, E., & Huh, Y.-M. (2013). Aptamer-modified magnetic nanoprobe for molecular MR imaging of VEGFR2 on angiogenic vasculature. Nanoscale Research Letters, 8(1), 399.
Jana, J., Lee, H. J., Chung, J. S., Kim, M. H., & Hur, S. H. (2019). Blue emitting nitrogen-doped carbon dots as a fluorescent probe for nitrite ion sensing and cell-imaging. Analytica Chimica Acta, 1079, 212–219. https://doi.org/10.1016/j.aca.2019.06.064.
Wolfbeis, O. S. (2015). An overview of nanoparticles commonly used in fluorescent bioimaging. Chemical Society Reviews, 44(14), 4743–4768. https://doi.org/10.1039/c4cs00392f.
Naseri, N., Ajorlou, E., Asghari, F., & Pilehvar-Soltanahmadi, Y. (2018). An update on nanoparticle-based contrast agents in medical imaging. Artificial Cells, Nanomedicine and Biotechnology, 46(6), 1111–1121. https://doi.org/10.1080/21691401.2017.1379014.
Sharma, A., & Das, J. (2019). Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging, and biomedicine. Journal of nanobiotechnology, 17(1), 92.
Unnikrishnan, B., Wu, R. S., Wei, S. C., Huang, C. C., & Chang, H. T. (2020). Fluorescent carbon dots for selective labeling of subcellular organelles. ACS Omega, 5(20), 11248–11261. https://doi.org/10.1021/acsomega.9b04301.
Doñate-Buendia, C., Torres-Mendieta, R., Pyatenko, A., Falomir, E., Fernández-Alonso, M., & Mínguez-Vega, G. (2018). Fabrication by laser irradiation in a continuous flow jet of carbon quantum dots for fluorescence imaging. ACS Omega, 3(3), 2735–2742. https://doi.org/10.1021/acsomega.7b02082.
Ganguly, S., Das, P., Itzhaki, E., Hadad, E., Gedanken, A., & Margel, S. (2020). Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Applied Materials and Interfaces, 12(46), 51940–51951. https://doi.org/10.1021/acsami.0c14527.
Hou, Y., Lu, Q., Deng, J., Li, H., & Zhang, Y. (2015). One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Analytica Chimica Acta, 866, 69–74. https://doi.org/10.1016/j.aca.2015.01.039.
Shang, W., Cai, T., Zhang, Y., Liu, D., & Liu, S. (2018). Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: all carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribology International, 118, 373–380. https://doi.org/10.1016/j.triboint.2017.09.029.
Kim, S., Song, Y., & Heller, M. J. (2017). Seamless aqueous arc discharge process for producing graphitic carbon nanostructures. Carbon, 120, 83–88. https://doi.org/10.1016/j.carbon.2017.04.006.
Wu, Y., Liu, Y., Yin, J., Li, H., & Huang, J. (2019). Facile ultrasonic synthesized NH2-carbon quantum dots for ultrasensitive Co2+ ion detection and cell imaging. Talanta, 205(May), 120121 https://doi.org/10.1016/j.talanta.2019.120121.
Hashemi, F., Heidari, F., Mohajeri, N., Mahmoodzadeh, F., & Zarghami, N. (2020). Fluorescence intensity enhancement of green carbon dots: synthesis, characterization and cell imaging. Photochemistry and Photobiology, 96(5), 1032–1040. https://doi.org/10.1111/php.13261.
Shao, Y., Zhu, C., Fu, Z., Lin, K., Wang, Y., Chang, Y., Tian, F. (2020). Green synthesis of multifunctional fluorescent carbon dots from mulberry leaves (Morus alba L.) residues for simultaneous intracellular imaging and drug delivery. Journal of Nanoparticle Research, 22(8). https://doi.org/10.1007/s11051-020-04917-4.
Radnia, F., Mohajeri, N., & Zarghami, N. (2020). New insight into the engineering of green carbon dots: Possible applications in emerging cancer theranostics. Talanta: Elsevier. 10.1016/j.talanta.2019.120547.
Mohajeri, N., Mostafavi, E., & Zarghami, N. (2020). The feasibility and usability of DNA-dot bioconjugation to antibody for targeted in vitro cancer cell fluorescence imaging. Journal of Photochemistry and Photobiology B: Biology, 209. https://doi.org/10.1016/j.jphotobiol.2020.111944.
Van Simaeys, D., López-Colón, D., Sefah, K., Sutphen, R., Jimenez, E., & Tan, W. (2010). Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PloS one, 5(11), e13770 https://doi.org/10.1371/journal.pone.0013770.
Scoville, D. J., Uhm, T. K. B., Shallcross, J. A., & Whelan, R. J. (2017). Selection of DNA aptamers for ovarian cancer biomarker CA125 using one-pot SELEX and high-throughput sequencing. Journal of nucleic acids, 2017. https://doi.org/10.1155/2017/9879135.
Gedi, V., Song, C. K., Kim, G. B., Lee, J. O., Oh, E., Shin, B. S., & Kim, Y.-P. (2018). Sensitive on-chip detection of cancer antigen 125 using a DNA aptamer/carbon nanotube network platform. Sensors and Actuators B: Chemical, 256, 89–97. https://doi.org/10.1016/j.snb.2017.10.049.
Nimith, K. M., Satyanarayan, M. N., & Umesh, G. (2018). Enhancement in fluorescence quantum yield of MEH-PPV:BT blends for polymer light emitting diode applications. Optical Materials, 80(April), 143–148. https://doi.org/10.1016/j.optmat.2018.04.046.
Kloudová, K., Hromádková, H., Partlová, S., Brtnický, T., Rob, L., Bartůňková, J., & Fialová, A. (2016). Expression of tumor antigens on primary ovarian cancer cells compared to established ovarian cancer cell lines. Oncotarget, 7(29), 46120 https://doi.org/10.18632/oncotarget.10028.
Radnia, F., Mohajeri, N., Hashemi, F., Imani, M., & Zarghami, N. (2021). Design and development of folate-chitosan/CD nanogel: An efficient fluorescent platform for Cancer-specific delivery of AntimiR-21. Reactive and Functional Polymers, 104814. https://doi.org/10.1016/j.reactfunctpolym.2021.104814.
Ţucureanu, V., Matei, A., Avram, A. M., Ţucureanu, V., Matei, A., Marius, A., & Avram, A. M. (2016). Critical reviews in analytical chemistry FTIR spectroscopy for carbon family study FTIR spectroscopy for carbon family study. Critical reviews in analytical chemistry, 46(6), 502–520. https://doi.org/10.1080/10408347.2016.1157013.
Hermanson, G. T. (2013). Bioconjugate Techniquese (3rd ed.). Elsevier Inc.
Ma, X., Song, S., Kim, S., Kwon, M., Lee, H., Park, W., & Sim, S. J. (2019). Single gold-bridged nanoprobes for identification of single point DNA mutations. Nature Communications, 10(1), 836 https://doi.org/10.1038/s41467-019-08769-y.
Wang, B., Chen, Y., Wu, Y., Weng, B., Liu, Y., Lu, Z., & Yu, C. (2016). Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosensors and Bioelectronics, 78, 23–30. https://doi.org/10.1016/j.bios.2015.11.015.
Shokri, E., Hosseini, M., Davari, M. D., Ganjali, M. R., Peppelenbosch, M. P., & Rezaee, F. (2017). Disulfide-induced self-assembled targets: a novel strategy for the label free colorimetric detection of DNAs/RNAs via unmodified gold nanoparticles. Scientific reports, 7(Mar), 45837 https://doi.org/10.1038/srep45837.
Yuanyuan L, Liping J, Bijun L, Xinyue F, Wei W, Pingping L, Shenghao X & Luo, X. (2019). Nitrogen doped carbon dots: mechanism investigation and their application for label free CA125 analysis. Journal of Materials Chemistry B. https://doi.org/10.1039/C9TB00021F.
Kurt, H., Yüce, M., Hussain, B., & Budak, H. (2016). Dual-excitation upconverting nanoparticle and quantum dot aptasensor for multiplexed food pathogen detection. Biosensors and Bioelectronics, 81, 280–286. https://doi.org/10.1016/j.bios.2016.03.005.
Vasimalai, N., Vilas-Boas, V., Gallo, J., de Fátima Cerqueira, M., Menéndez-Miranda, M., Costa-Fernández, J. M., & Fernández-Argüelles, M. T. (2018). Green synthesis of fluorescent carbon dots from spices for in vitro imaging and tumour cell growth inhibition. Beilstein journal of nanotechnology, 9(1), 530–544. https://doi.org/10.3762/bjnano.9.51.
Torchynska, T. V., Vorobiev, Y. V., Makhniy, V. P., & Horley, P. P. (2014). The influence of bio-conjugation on photoluminescence of CdSe/ZnS quantum dots. Physica B: Condensed Matter, 453(i), 68–71. https://doi.org/10.1016/j.physb.2014.05.026.
Brownell, L. V., Robins, K. A., Jeong, Y., Lee, Y., & Lee, D.-C. (2013). Highly systematic and efficient HOMO–LUMO energy gap control of thiophene-pyrazine-acenes. The Journal of Physical Chemistry C, 117(48), 25236–25247. https://doi.org/10.1021/jp407269p.
Miao, H., Wang, L., Zhuo, Y., Zhou, Z., & Yang, X. (2016). Label-free fluorimetric detection of CEA using carbon dots derived from tomato juice. Biosensors and Bioelectronics, 86, 83–89. https://doi.org/10.1016/j.bios.2016.06.043.
Chem, J. M. (2012). Electron transfer quenching by nitroxide radicals of the fluorescence of carbon dots †. Journal of Materials Chemistry, 11801–11807. https://doi.org/10.1039/c2jm31191g.
Zu, F., Yan, F., Bai, Z., Xu, J., Wang, Y., Huang, Y., & Zhou, X. (2017). The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchimica Acta, 184(7), 1899–1914. https://doi.org/10.1007/s00604-017-2318-9.
Srivastava, I., Misra, S. K., Bangru, S., Boateng, K. A., Soares, J. A. N. T., Schwartz-Duval, A. S., & Pan, D. (2020). Complementary oligonucleotide conjugated multicolor carbon dots for intracellular recognition of biological events. ACS Applied Materials and Interfaces, 12(14), 16137–16149. https://doi.org/10.1021/acsami.0c02463.
Ashwood, B., Sanstead, P. J., Dai, Q., He, C., & Tokmakoff, A. (2020). 5-carboxylcytosine and cytosine protonation distinctly alter the stability and dehybridization dynamics of the DNA duplex. Journal of Physical Chemistry B, 124(4), 627–640. https://doi.org/10.1021/acs.jpcb.9b11510.
Zhang, J., Lang, H. P., Yoshikawa, G., & Gerber, C. (2012). Optimization of DNA hybridization efficiency by pH-driven nanomechanical bending. Langmuir, 28(15), 6494–6501. https://doi.org/10.1021/la205066h.
Takezawa, Y., & Shionoya, M. (2012). Metal-mediated DNA base pairing: alternatives to hydrogen-bonded Watson-Crick base pairs. Accounts of Chemical Research, 45(12), 2066–2076. https://doi.org/10.1021/ar200313h.
Hong, L., Lu, M., Dinel, M.-P., Blain, P., Peng, W., Gu, H., & Masson, J.-F. (2018). Hybridization conditions of oligonucleotide-capped gold nanoparticles for SPR sensing of microRNA. Biosensors and Bioelectronic, 109, 230–236. https://doi.org/10.1016/j.bios.2018.03.032.
Robert E. Farrell, J. (2017). RNA methodologies: a laboratory guide for isolation and characterization. FEBS Letters (5rd ed., Vol. 331). Academic Press.
Rahaie, M., Naghavi, M. R., Alizadeh, H., Malboobi, M. A., & Dimitrov, K. (2011). A novel DNA-based nanostructure for single molecule detection purposes. International Journal of Nanotechnology, 8(6–7), 458–470. https://doi.org/10.1504/IJNT.2011.040188.
Yang, T., & Gao, H. (2019). Atomic force microscopy study of EDTA induced desorption of metal ions immobilized DNA from mica surface. Ultramicroscopy, 199(Jan), 7–15. https://doi.org/10.1016/j.ultramic.2019.01.001.
Pelaz, B., Alexiou, C., Alvarez-Puebla, R. A., Alves, F., Andrews, A. M., Ashraf, S., & Parak, W. J. (2017). Diverse applications of nanomedicine. ACS Nano, 11(3), 2313–2381. https://doi.org/10.1021/acsnano.6b06040.
Long, F., Wu, S., He, M., Tong, T., & Shi, H. (2011). Ultrasensitive quantum dots-based DNA detection and hybridization kinetics analysis with evanescent wave biosensing platform. Biosensors and Bioelectronics, 26(5), 2390–2395. https://doi.org/10.1016/j.bios.2010.10.018.
Acknowledgements
The authors would like to thank Tabriz University of Medical Sciences (TUOMS) for financially supporting this project. This study was partly taken from the MSc thesis (IR.TBZMED.VCR.REC.1397.114) that performed at the Faculty of Advanced Medical Sciences of Tabriz University of Medical Science, Tabriz, Iran.
Author contributions
All the authors contributed equally.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved by the Local Research ethical committee of TUOMS.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heidari, F., Mohajeri, N. & Zarghami, N. Targeted design of green carbon dot-CA-125 aptamer conjugate for the fluorescence imaging of ovarian cancer cell. Cell Biochem Biophys 80, 75–88 (2022). https://doi.org/10.1007/s12013-021-01034-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-01034-4