Abstract
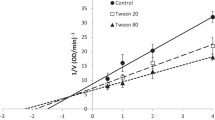
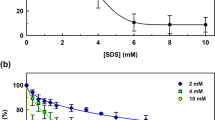
In this study, we have reported the effect of nonionic, anionic, cationic, and zwitterionic detergents on the enzymatic activity and structural stability of Rhizopus niveus lipase. Secondary structural changes were monitored by Far-UV CD which shows that surfactant induces helicity in the Rhizopus niveus lipase protein which was maximum in case of CTAB followed by SDS, CHAPS, and Brij-35. Similarly, tertiary structural changes were monitored by tryptophan fluorescence. We also carried out enzyme kinetics assays which showed that activity was enhanced by 1.5- and 1.1-fold in the presence of CHAPS and Brij-35, respectively. Furthermore, there was a decline in activity by 20 and 30 % in case of SDS and CTAB, respectively. These studies may be helpful in understanding detergent–lipase interaction in greater detail as lipases are used in many industrial processes.




Similar content being viewed by others
References
Bhardwaj, K., Raju, A., & Rajasekharan, R. (2000). Identification, purification and characterization of thermally stable lipase from rice bran, a new member of the (phospho) lipase family. Plant Physiology, 127, 1728–1738.
Carrière, F., Thirstrup, K., Hjorth, S., & Boel, E. (1994). Cloning of the classical guinea pig pancreatic lipase and comparison with lipase related protein 2. FEBS Letters, 338, 63–68.
Olempska-Beer, Z. S., Merker, R. I., Ditto, M. D., & DiNovi, M. J. (2006). Food processing enzymes from recombinant microorganism: A review. Regulatory Toxicology and Pharmacology, 45, 144–158.
Hasan, F., Shah, A. A., & Hameed, A. (2006). Industrial applications of microbial lipases. Enzyme and Microbial Technology, 39, 235–251.
Rahman, R. N., Baharum, S. N., Basri, M., & Salleh, A. B. (2005). High-yielding purification of an organic solvent-tolerant lipase from Pseudomonas sp. Analytical Biochemistry, 341, 267–274.
Villeneuve, P., & Foglia, T. (1997). Lipase specificities: Potential applications in lipid bioconversions. Inform, 8, 640–650.
Saxena, R. K., Sheoran, A., Giri, B., & Davidson, W. S. (2003). Purification strategies for microbial lipases. Journal of Microbiol Methods, 52, 1–18.
Li, H., & Zhang, X. (2005). Characterization of thermostable lipase from thermophilic Geobacillus sp. TW1. Protein Expression and Purification, 42, 153–159.
Ollis, D. L., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S. M., et al. (1992). The alpha/beta hydrolase fold. Protein Engineering, 5, 197–211.
Svendsen, A. (2000). Lipase protein engineering. Biochimica et Biophysica Acta, 1543, 223–238.
Beer, H. D., McCathy, J. E., Borncheuer, U. T., & Schmidit, R. D. (1998). Cloning, expression, characterization and role of leader sequence of lipase from Rhizopusoryzae. Biochimica et Biophysica Acta, 1399, 173–180.
Kohno, M., Kugimiya, W., Hashimoto, Y., & Morita, Y. (1994). Purification, characterization and crystallisation of two types of lipase from Rhizopus niveus. Bioscience, Biotechnology, and Biochemistry, 58, 1007–1012.
Ben, Salah A., Sayarri, A., Verger, R., & Gargouri, Y. (2001). Kinetic study of Rhizopusoryzae using monomolecular film technique. Biochemie, 83, 463–493.
Reis, P., Holmberg, K., Watzke, H., Leser, M. E., & Miller, R. (2009). Lipases at interfaces: A review. Advances in Colloid and Interface Science, 148, 237–250.
Borgstrom, B., & Donnér, J. (1976). Interaction of pancreatic lipase with bile salts and dodecyl sulphate. Lipid Research, 17, 491–507.
Cherif, S., Mnif, S., Hadrich, F., Abdelkafi, F., & Sayadi, S. (2011). A newly highly alkaline lipase: An ideal choice for application in detergent formulations. Lipids in Health and Disease, 10, 221.
Chen, Y. H., Yang, J. T., & Martinez, H. (1972). Determination of the secondary structures of proteins by circular dichroism and optical rotator dispersion. Biochemistry, 11, 4120–4131.
Andrade, M. A., Chacón, P., Merelo, J. J., & Morán, F. (1993). Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsurprised learning neural network. Protein Engineering, 6, 383–390.
Naeem, A., Fatima, S., & Khan, R. H. (2006). Characterization of partially folded intermediates of papain in presence of cationic, anionic and nonionic detergents at low pH. Biopolymers, 83(1), 1–10.
Lakowicz, J. R. (1992). Topics in fluorescence spectroscopy: Biological applications (Vol. 3, pp. 289–343). New York: Plenum Press.
Gasymov, O. K., & Glasgow, B. J. (2007). ANS fluorescence: potential to augment the identification of the external binding sites of proteins. Biochimica et Biophysica Acta, 1774, 403–411.
Ptisyn, O. B. (1992). The molten globule State. In T. E. Creighton (Ed.), Protein folding (pp. 243–300). New York: W. H. freeman.
Mogensen, J. E., Sehgal, P., & Otzen, D. E. (2005). Activation, inhibition and destabilization of Thermomyceslanuginosus lipase by detergents. Biochemistry, 44, 1719–1730.
Andreas, B., Tobias, R., Matthias, H., Winfried, H., Jochen, B., & Marion, A. S. (2005). pH optima in lipase catalysed esterification. Biocatalysis and Biotransformation, 23, 307–314.
Paiva, A. L., Balca, V. M., & Malacta, F. X. (2000). Kinetics and mechanism of reactions catalysed by immobilized lipases. Enzyme and Microbial Technology, 27, 187–204.
Martinell, M., Holmquist, M., & Hulk, K. (1995). On the interfacial activation of Candida Antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochimica et Biophysica Acta, 1258, 272–276.
Hermoso, J., Pignol, D., Kerfelec, B., Crenon, I., Chapus, C., & Fontecilla-Camps, J.-C. (1996). Lipase activation by non-ionic detergents, The crystal structure of the porcine lipase–colipasetetraethylene glycol monooctyl ether complex. Protein Journal of Biological Chemistry, 271, 8007–80016.
Antonov, V. K., Dyakov, V. L., Mishin, A. A., & Rotanovo, T. V. (1998). Catalytic activity and association of pancreatic lipase. Biochimie, 70, 1235–1244.
Ruiz, C., Falcocchio, S., Xoxi, E., Pastor, F. I., Diaz, P., & Saso, L. (2004). Activation and inhibition of Candida rugosa and Bacillus- related lipases by saturated fatty acids evaluated by new calorimetric microassay. Biochimica et Biophysica Acta, 1672, 184–191.
Acknowledgments
Authors are thankful for the financial support from Council for Scientific and Industrial Research (PA is recipient of CSIR-JRF and GR is recipient of CSIR-SRF), New Delhi and the Central facility of Interdisciplinary Biotechnology Unit, AMU Aligarh.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, P., Rabbani, G., Badr, G. et al. The Surfactant-Induced Conformational and Activity Alterations in Rhizopus niveus Lipase. Cell Biochem Biophys 71, 1199–1206 (2015). https://doi.org/10.1007/s12013-014-0329-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0329-2