Abstract
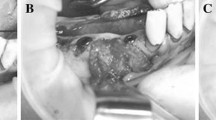
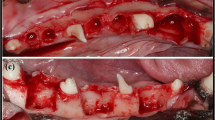
Guided bone regeneration (GBR) is a principle adopted from guided tissue regeneration (GTR). Wherein, GBR is used for the healing of peri-implant bony dehiscences, for the immediate placement of implants into extraction sockets and for the augmentation of atrophic alveolar ridges. This procedure is done by the placement of a resorbable or non-resorbable membrane that will exclude undesirable types of tissue growth between the extraction socket and the soft tissue to allow only bone cells to regenerate in the surgically treated lesion. Here, we investigated the biodegradable effect of polylactic-co-glycolic acid (PLGA) membrane in the alveolar bone on Beagle dogs. Results show that both collagen and PLGA membrane had been fully resorbed, biodegraded, at four weeks post-operative reentry into the alveolar bone. Histological results under light microscopy revealed formation of new bone trabeculae in the extraction sites on both collagen and PLGA membrane. In conclusion, PLGA membrane could be a potential biomaterials for use on GBR and GTR. Nevertheless, further studies will be necessary to elucidate the efficiency and cost effectiveness of PLGA as GBR membrane in clinical.




Similar content being viewed by others
References
Lindhe, J. (1997). Clinical periodontology and implant dentistry (3rd ed., pp. 597, 906, 913, 914). Denmark: Munksgaard Intl Pub.
Hurzeler, M. B., Quinones, C. R., Hutmacher, D., & Schupbach, P. (1997). Guided bone regeneration around dental implants in the atrophic alveolar ridge using a bioresorbable barrier. An experimental study in the monkey. Clinical Oral Implants Research, 8, 323–331.
Dimitriou, R., Mataliotakis, G. I., Calori, G. M., & Giannoudis, P. V. (2012). The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Medicine, 10, 81.
Hutmacher, D., Hurzeler, M. B., & Schliephake, H. (1996). A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. International Journal of Oral and Maxillofacial Implants, 11(5), 667–678.
Schmitt, E. F., & Palistina, R. A. (1967). 1969, 1973. Patent no. 3.371.069, 3.463.158, 3739.773. US.
Kucukkobasi, H., Mutlu, N., Isik, K., Celik, I., et al. (2009). Histological evaluation of the effects of bioglass, hydroxyapatite, or demineralized freeze-dried bone, grafted alone or as composites, on the healing of tibial defects in rabbits. Saudi Medical Journal, 30, 329.
Wang, X., & Li, X. (2008). Progress of researches on guided bone regeneration membrane. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi, 25, 941.
Lundgren, D., Nyman, S., Mathsen, T., Isaksson, S., et al. (1992). Guided bone regeneration of cranial defect, using biodegradable barriers: An experimental pilot study in the rabbit. Journal of Cranio-Maxillo-Facial Surgery, 20, 257.
Winet, H., & Hollinger, J. O. (1993). Incorporation of polylactide-polyglycolide in a cortical defect: neoosteogenesis in a bone chamber. Journal of Biomedical Materials Research, 27, 667.
Miyamoto, S., Takoaka, K., & Ono, K. (1993). Bone induction and bone repair by composites of bone morphogenetic protein and biodegradable synthetic polymers. Annales Chirurgiae et Gynaecologiae Supplementum, 1993(207), 69–75.
Cauwels, R. G., & Martens, L. C. (2004). Use of osteoconductive materials in pediatric dental medicine. Revue Belge de Medecine Dentaire, 59, 203.
Lü, J.-M., Wang, X., Marin-Muller, C., Wang, H., Lin, P. H., Yao, Q., et al. (2009). Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Review of Molecular Diagnostics, 9(4), 325–341.
Makadia, H. K., & Siegel, S. J. (2011). Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers, 3, 1377–1397.
Allison, S. D. (2008). Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic) acid microparticle drug delivery systems. Journal of Pharmaceutical Sciences, 97, 2022–2035.
Mundargi, R., Babu, V., Rangaswamy, V., Patel, P., & Aminabhavi, T. (2008). Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. Journal of Controlled Release, 125, 193–209.
Mohamed, F., & van der Walle, C. F. (2008). Engineering biodegradable polyester particles with specific drug targeting and drug release properties. Journal of Pharmaceutical Sciences, 97, 71–87.
Anderson, J. M., & Shive, M. S. (1997). Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews, 28, 5–24.
Wake, M. C., Gerecht, P. D., Liu, L. C., et al. (1998). Effect of biodegradable polymer particles on rat marrow-derived stromal osteoblast in vitro. Journal of Biomaterials, 19, 1255–1268.
Catelas, I., Petit, A., Zukor, D. J., et al. (1999). Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Journal of Biomaterials, 20, 625–630.
Jiao, S. (2003). Biological evaluations of bio-materials and medical instruments. Chi J Med Inst, 27(1), 1–4.
Carlino, P., Pepe, V., Pollice, G., & Grassi, F. R. (2008). Immediate transmucosal implant placement in fresh maxillary and mandibular molar extraction sockets: Description of technique and preliminary results. Minerva Stomatologica, 57, 471.
Kao, S. T., & Scott, D. D. (2007). A review of bone substitutes. Oral and Maxillofacial Surgery Clinics of North America, 19, 513.
Buser, D., Dula, K., Belser, H., Hirt, H. P., & Berthold, H. (1995). Localized ridge augmentation using guided bone regeneration. II. Surgical procedures in the mandible. International Journal of Periodontics and Restorative Dentistry, 15, 13–29.
Lang, N. P., Becker, W., & Karring, T. (2000). Alveolar bone formation. In J. Lindhe (Ed.), Clinical periodontology and implant dentistry (3rd ed., pp. 906–937). Copenhagen: Munksgaard.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hua, N., Ti, V.L. & Xu, Y. Biodegradable Effect of PLGA Membrane in Alveolar Bone Regeneration on Beagle Dog. Cell Biochem Biophys 70, 1051–1055 (2014). https://doi.org/10.1007/s12013-014-0022-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0022-5