Abstract
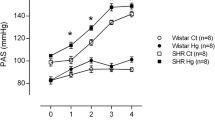
Mercury is considered a risk factor for the development of hypertension and other cardiovascular diseases. We investigated whether the effects of mercury exposure on haemodynamic parameters of young Wistar rats and prehypertensive SHRs might alter the time course of hypertension development. Young (4 weeks) male Wistar rats and SHRs were randomly assigned to four groups: untreated Wistar rats (Wistar Ct), Wistar rats exposed to mercury chloride for 30 days (Wistar Hg), untreated SHRs (SHR Ct) and SHRs exposed to mercury chloride (SHR Hg) for 30 days. Non-invasive and invasive arterial pressures were measured to investigate pressure reactivity; nitrite/nitrate levels, ACE activity, and lipid peroxidation were measured in plasma. The systolic blood pressure (SBP) of the Wistar rat groups did not change but increased in the SHRs from the second week to the last week. Hg exposure accelerated the increase in the SBP of SHRs. L-NAME administration increased SBP and diastolic blood pressure (DBP) in all groups, but this increase was smaller in SHRs exposed to Hg. A decrease in plasma nitrite and nitrate levels in the SHR Hg group suggested that mercury reduced NO bioavailability. Tempol-reduced blood pressure suggesting that the superoxide anion played a role in the marked increase in this parameter. These findings provide evidence that Hg exposure might activate mechanisms to accelerate hypertension development, including a reduction in NO bioavailability. Therefore, predisposed individuals under mercury exposure are at greater risk from an enhanced development of hypertension.








Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
References
Agency for Toxic Substances and Disease Registry (ATSDR). (1999). Toxicological profile for mercury. ATSDR, Public Health Service, US Department of health and human services: 1999. Retrieved September 10, 2021, from http://www.atsdr.cdc.gov/csem/pediatric/appendixb.html.
Yang, L., Zhang, Y., Wang, F., Luo, Z., Guo, S., & Strähle, U. (2020). Toxicity of mercury: Molecular evidence. Chemosphere, 245, 125586. https://doi.org/10.1016/j.chemosphere.2019.125586
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. Experientia Supplementum, 101, 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Agency for Toxic Substances and Disease Registry (ATSDR). (2015). Priority list of hazardous substances. Retrieved from http://www.atsdr.cdc.gov/spl/index.html#modalIdString_myTable2015.
Salonen, J. T., Seppanen, K., Lakka, T. A., Salonen, R., & Kaplan, G. A. (2000). Mercury accumulation and accelerated progression of carotid atherosclerosis: A population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis, 148(2), 265–273. https://doi.org/10.1016/S0021-9150(99)00272-5
Guallar, E., Sanz-Gallardo, I., Veer, P. V., Bode, P., Aro, A., Gomes-Aracema, J., Kark, J. D., Riemersma, R. A., Martín-Moreno, J. M., & Kok, F. J. (2002). Mercury, fish oils, and the risk of myocardial infarction. The New England Journal of Medicine, 347(22), 1747–1754. https://doi.org/10.1056/NEJMoa020157
Houston, M. C. (2007). The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease and myocardial infarction. Alternative Therapies in Health and Medicine, 13(2), 128–133.
Virtanen, J. K., Voutilainen, S., Rissanen, T. H., Mursu, J., Tuomainen, T. P., Korhonen, M. J., Valkonen, V. P., Seppänen, K., Laukkanen, J. A., & Salonen, J. T. (2005). Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arteriosclerosis, Thrombosis and Vascular Biology, 25(1), 228–233. https://doi.org/10.1161/01.ATV.0000150040.20950.61
Virtanen, J. K., Rissanen, T. H., Voutilainen, S., & Tuomainen, T. P. J. (2007). Mercury as a risk factor for cardiovascular diseases. The Journal of Nutritional Biochemistry, 18(2), 75–85. https://doi.org/10.1016/j.jnutbio.2006.05.001
Choi, A. L., Weihe, P., Budtz-Jorgensen, E., Jorgensen, P. J., Salonen, J. T., Tuomainen, T. P., Murata, K., Nielsen, H. P., Petersen, M. S., Askham, J., & Grandjean, P. (2009). Methyl mercury exposure and adverse cardiovascular effects in Faroese whaling men. Environmental Health Perspectives, 117(3), 367–372. https://doi.org/10.1289/ehp.11608
Vassallo, D. V., Simões, M. R., Furieri, L. B., Fioresi, M., Fiorim, J., Almeida, E. A. S., Angeli, J. K., Wiggers, G. A., Peçanha, F. M., & Salaices, M. (2011). Toxic effects of mercury, lead and gadolinium on vascular reactivity. Brazilian Journal of Medical and Biological Research, 44(9), 939–946. https://doi.org/10.1590/S0100-879X2011007500098
Genchi, G., Sinicropi, M. S., Carocci, A., Lauria, G., & Catalano, A. (2017). Mercury exposure and heart diseases. International Journal of Environmental Research and Public Health, 14(1), 74. https://doi.org/10.3390/ijerph14010074
Vassallo, D. V., Wiggers, G. A., Padilha, A. S., & Ronacher, S. M. (2020). Endothelium: A target for harmful actions of metals. Current Hypertension Reviews, 16(3), 201–209. https://doi.org/10.2174/1573402115666190115153759
Hu, X. F., Lowe, M., & Chan, H. M. (2021). Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environmental research, 193, 110538. https://doi.org/10.1016/j.envres.2020.110538
Langworth, S., Sallsten, G., Barregard, L., Cynkier, I., Lind, M. L., & Soderman, E. (1997). Exposure to mercury vapour and impact on health in the dental profession in Sweden. Journal of Dental Research, 76(7), 1397–1404. https://doi.org/10.1177/00220345970760071001
Clarkson, T. W. (1972). The pharmacology of mercury compounds. Annual Review of Pharmacology, 12, 375–406. https://doi.org/10.1146/annurev.pa.12.040172.002111
Clarkson, T. W., Magos, L., & Myers, G. J. (2003). The toxicology of mercury—current exposures and clinical manifestations. The New England Journal of Medicine, 349(18), 1731–1737. https://doi.org/10.1056/nejmra022471
Clifton, J. C. (2007). Mercury exposure and public health. Pediatric Clinics of North America, 54(2), 237–269. https://doi.org/10.1016/j.pcl.2007.02.005
Maqbool, F., Niaz, K., Hassan, F. I., Khan, F., & Abdollahi, M. (2017). Immunotoxicity of mercury: Pathological and toxicological effects. Journal of Environmetal Science and Health Part C, 35(1), 29–46. https://doi.org/10.1080/10590501.2016.1278299
Goyer, R. A. (1997). Toxic and essential metal interactions. Annual Review of Nutrition, 17, 37–50. https://doi.org/10.1146/annurev.nutr.17.1.37
Carmignani, M., & Boscolo, P. (1984). Cardiovascular Homeostasis in rats chronically exposed to mercury chloride. Archives of Toxicology, 7, 383–388. https://doi.org/10.1007/978-3-642-69132-4_66
Wiggers, G. A., Peçanha, F. M., Briones, A. M., Pérez-Girón, J. V., Miguel, M., Vassallo, D. V., Cachofeiro, V., Alonso, M. J., & Salaices, M. (2008). Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. American Journal of Physiology, Heart and Circulatory Physiology, 295(3), 1033–1043. https://doi.org/10.1152/ajpheart.00430.2008
Rizzetti, D. A., Torres, J. G., Escobar, A. G., da Silva, T. M., Moraes, P. Z., Hernanz, R., Peçanha, F. M., Castro, M. M., Vassallo, D. V., Salaices, M., Alonso, M. J., & Wiggers, G. A. (2017). The cessation of the long-term exposure to low doses of mercury ameliorates the increase in systolic blood pressure and vascular damage in rats. Environmental Research, 155, 182–192. https://doi.org/10.1015/j.envres.2017.02.022
Rizzetti, D. A., Silva, T. M., Escobar, A. G., Piagette, J., Peçanha, F. M., Vassallo, D. V., Alonso, M. J., Salaices, M., & Wiggers, G. A. (2018). Mercury-induced vascular dysfunction is mediated by angiotensin II AT-1 receptor upregulation. Environmental Research, 162, 287–296. https://doi.org/10.1016/j.envres.2018.01.026
Peçanha, F. M., Wiggers, G. A., Briones, A. M., Perez-Giron, J. V., Miguel, M., Garcia Redondo, A. B., Vassallo, D. V., Alonso, M. J., & Salaices, M. (2010). The role of cyclooxygenase (COX)-2 derived prostanoids on vasoconstrictor responses to phenylephrine is increased by exposure to low mercury concentration. Journal of Physiology and Pharmacology, 61(1), 29–36.
Simões, R. P., Fardin, P. B. A., Simões, M. R., Vassallo, D. V., & Padilha, A. S. (2020). Long-term mercury exposure accelerates the development of hypertension in prehypertensive spontaneously hypertensive rats inducing endothelial dysufnction: The role of oxidative stress and cyclooxygenase-2. Biological Trace Element Research, 196(2), 565–578. https://doi.org/10.1007/s12011-019-01952-8
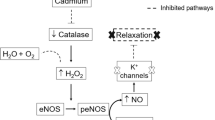
Fardin, P. B. A., Simões, R. P., Schereider, I. R. G., Almenara, C. C. P., Simões, M. R., & Vassallo, D. V. (2020). Chronic mercury exposure in prehypertensive SHRs accelerates hypertension development and activates vasoprotective mechanisms by increasing NO and H2O2 production. Cardiovascular Toxicology, 20(3), 197–210. https://doi.org/10.1007/s12012-019-09545-6
Bassaneze, V., Barauna, V. G., Lavini-Ramos, C., Kalil, J., Schettert, I. T., Miyakawa, A. A., & Krieger, J. E. (2009). Shear stress induces nitric oxide-mediated vascular endothelial growth factor production in human adipose tissue mesenchymal stem cells. Stem Cells and Development, 19(3), 371–378. https://doi.org/10.1089/scd.2009.0195
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Furieri, L. B., Galán, M., Avendaño, M. S., García-Redondo, A. B., Aguado, A., Martínez, S., Cachofeiro, V., Bartolomé, M. V., Alonso, M. J., Vassallo, D. V., & Salaices, M. (2011). Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. British Journal of Pharmacology, 162(8), 1819–1831. https://doi.org/10.1111/j.1476-5381.2011.01203.x
Rizzetti, D. A., Torres, J. G. D., Escobar, A. G., Peçanha, F. M., Santos, F. W., Puntel, R. L., Alonso, M. J., Briones, A. M., Salaices, M., Vassallo, D. V., & Wiggers, G. A. (2013). Apocynin prevents vascular effects caused by chronic exposute to low concentrations of mercury. PLoS ONE, 8(2), e55806. https://doi.org/10.1371/journal.pone.0055806
Aguado, A., Galan, M., Zhenyukh, O., Wiggers, G. A., Roque, F. R., Redondo, S., Pecanha, F., Martin, A., Fortuno, A., Cachofeiro, V., Tejerina, T., Salaices, M., & Briones, A. M. (2013). Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase-2 pathways. Toxicology and Applied Pharmacology, 268(2), 188–200. https://doi.org/10.1016/j.taap.2013.01.030
Escobar, A. G., Rizzetti, D. A., Piagette, J. T., Peçanha, F. M., Vassallo, D. V., Miguel, M., & Wiggers, G. A. (2020). Antioxidant properties of egg white hydrolysate prevent mercury-induced vascular damage in resistance arteries. Frontiers in Physiology, 11, 595767. https://doi.org/10.3389/fphys.2020.595767
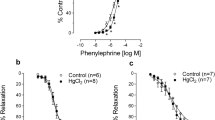
Machado, A. C., Padilha, A. S., Wiggers, G. A., Siman, F. D. M., Stefanon, I., & Vassallo, D. V. (2007). Small doses of mercury increase arterial pressure reactivity to phenylephrine in rats. Environmental Toxicology and Pharmacology, 24(2), 92–97. https://doi.org/10.1016/j.etap.2007.02.005
Botelho, T., Marques, V. B., Simões, M. R., do Val Lima, P. R., Simões, F. V., Vassallo, D. V., & Dos Santos, L. (2019). Impaired participation of potassium channels and Na+ /K+ -ATPase in vasodilatation due to reduced nitric oxide bioavailability in rats exposed to mercury. Basic & Clinical Pharmacology & Toxicology, 124(2), 190–198. https://doi.org/10.1111/bcpt.13113
Wolf, M. B., & Baynes, J. W. (2007). Cadmium and mercury cause an oxidative stress induced endothelial dysfunction. BioMetals, 20(1), 73–81. https://doi.org/10.1007/s10534-006-906-0
Wu, C. C., & Yen, M. H. (1999). Higher level of plasma nitric oxide in spontaneously hypertensive rats. American Journal of Hypertension, 12(5), 476–482. https://doi.org/10.1016/s0895-7061(99)00008-4
Ulker, S., Master, D., Keown, P. P., & Bayraktutan, U. (2003). Impaired activities of antioxidant enzymes elicit endothelial dysfunction in spontaneous hypertensive rats despite enhanced vascular nitric oxide generation. Cardiovascular Research, 59(2), 488–500. https://doi.org/10.1016/s0008-6363(03)00424-3
Cacanyiova, S., Cebova, M., Kunes, J., & Kristek, F. (2006). Comparison of vascular function and structure of iliac artery in spontaneously hypertensive and hereditary hypertriglyceridemic rats. Physiological Research, 55(1), 73–79.
Vaziri, N. D., Ni, Z., & Oveisi, F. (1998). Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension, 31(6), 1248–1254. https://doi.org/10.1161/01.hyp.31.6.124
Graham, D. A., & Rush, J. W. E. (2004). Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. Journal of Applied Physiology, 96(6), 2088–2096. https://doi.org/10.1152/japplphysiol.01252.2003
Chou, T. C., Yen, M. H., Li, C. Y., & Ding, Y. A. (1998). Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension, 31(2), 643–648. https://doi.org/10.1161/01.hyp.31.2.643
Tsikas, D. (2007). Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. Journal of Chromatography B Analytical Technology Biomedical Life Science, 851(1–2), 51–70. https://doi.org/10.1016/j.jchromb.2006.07.054
Förstermann, U. (2006). Janus-faced role of endothelial NO synthase in vascular disease: Uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biological Chemistry, 387(12), 1521–1533. https://doi.org/10.1515/bc.2006.190
Cordeiro, E. R., Filetti, F. M., Simões, M. R., & Vassallo, D. V. (2019). Mercury induces nuclear estrogen receptors to act as vasoconstrictors promoting endothelial denudation via the PI3K/Akt signaling pathway. Toxicology and Applied Pharmacology, 15(381), 114710. https://doi.org/10.1016/j.taap.2019.114710
Gillis, E. E., Brinson, K. N., Rafikova, O., Chen, W., Musall, J. B., Harrison, D. V., & Sullivan, J. C. (2018). Oxidative stress induces BH4 deficiency in male, but not female, SHR. Bioscience Reports. https://doi.org/10.1042/BSR20180111
Horvathova, M., Zitnanova, I., Kralovicova, Z., Balis, P., Puzserova, A., Muchova, J., Kluknavsky, M., Durackova, Z., & Bernatova, I. (2015). Sex differences in the blood antioxidant defense system in juvenile rats with various genetic predispositions to hypertension. Hypertension Research, 39(2), 64–69. https://doi.org/10.1038/hr.2015.117
Gutierrez, L. L. P., Mazzotti, N. G., Araújo, A. S. R., Klipel, R. B., Fernandes, T. R. G., Llesuy, S. F., & Belló-Klein, A. (2006). Peripheral markers of oxidative stress in chronic mercuric chloride intoxication. Brazilian Journal of Medical and Biological Research, 39, 767–772. https://doi.org/10.1590/S0100-879X2006000600009
Vassallo, D. V., Simões, M. R., Giuberti, K., Azevedo, B. F., Ribeiro Junior, R. F., Salaices, M., & Stefanon, I. (2019). Effects of chronic exposure to mercury on angiotensin-converting enzyme activity and oxidative stress in normotensive and hypertensive rats. Arquivos Brasileiros de Cardiologia, 112(4), 374–380. https://doi.org/10.5935/abc.20180271
Saleem, M., Pokkunuri, I., & Asghar, M. (2016). Superoxide increases angiotensin II AT1 receptor function in human kidney-2 cells. FEBS Open Bio, 6(12), 1273–1284. https://doi.org/10.1002/2211-5463.12148
Acknowledgements
This study was supported by Grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) 302968/2016-4 and PRONEX-CNPq/FAPES (Fundação de Amparo a Pesquisa do Espírito Santo) 80598773. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the submission of the manuscript to Cardiovascular Toxicology.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest related to the contents of this article to declare.
Ethical Approval
All experimental procedures were performed in accordance with the guidelines for the care and handling of laboratory animals recommended by the Brazilian Societies of Experimental Biology, and the study protocols were previously approved by the Ethics Committee in Animal Research of the Federal University of Espirito Santo (22/2018 CEUA-UFES).
Additional information
Handling Editor: Kurt Varner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ronchetti, G.Z., Simões, M.R., Schereider, I.R.G. et al. Oxidative Stress Induced by 30 Days of Mercury Exposure Accelerates Hypertension Development in Prehypertensive Young SHRs. Cardiovasc Toxicol 22, 929–939 (2022). https://doi.org/10.1007/s12012-022-09769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09769-z