Abstract
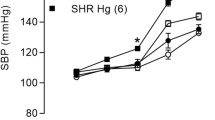
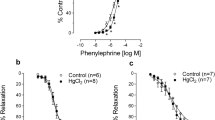
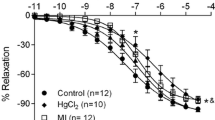
Mercury is a metal widely dispersed in nature that when in contact with human organism, it damages the cardiovascular system. Long-term mercury exposure for 30 days induces endothelial dysfunction without blood pressure changes in normotensive adult rats. However, it is not known whether exposure to mercury can exacerbate endothelial dysfunction and hypertension development in predisposed animals. Thus, we aimed to investigate the effects of long-term mercury exposure on the blood pressure (BP) and in the isolated aortas of young normotensive and prehypertensive spontaneously hypertensive rats (SHRs). Four-week-old male Wistar rats and SHRs were treated daily with mercury chloride (HgCl2) (1st dose, 4.6 μg/kg; subsequent dose, 0.07 μg/kg/day, im, 30 days) or vehicle. BP was assessed weekly and the vascular reactivity to phenylephrine was evaluated in isolated aorta from rats exposed or not to mercury. Mercury exposure did not affect BP in young Wistar rats but accelerated the development of hypertension in young SHRs. Vascular reactivity to phenylephrine increased only in the aorta from mercury-exposed SHRs. While HgCl2 exposure in SHRs did not alter nitric oxide production, we observed increased superoxide anion production and decreased superoxide dismutase-1 protein expression, and enhanced cyclooxygenase-2 (COX-2) participation with increased prostaglandin (PGE2) production and decreased prostacyclin. In the Wistar group, mercury exposure did not alter superoxide anion production or the COX-2 pathway. Mercury exposure accelerated the natural course of hypertension in young SHRs and increased oxidative stress associated with reduced participation of antioxidant enzymes, an activated COX-2 pathway, thereby producing endothelial dysfunction, which is a risk factor in prehypertensive individuals.







Similar content being viewed by others
References
Guzzi G, La Porta CAM (2008) Molecular mechanisms triggered by mercury. Toxicology 244:1–12. https://doi.org/10.1016/j.tox.2007.11.002
Vassallo DV, Simões MR, Furieri LB et al (2011) Toxic effects of mercury, lead and gadolinium on vascular reactivity. Brazilian J Med Biol Res 44:939–946. https://doi.org/10.1590/S0100-879X2011007500098
Mortazavi SMJ, Mortazavi G, Paknahad M (2016) A review on the distribution of Hg in the environment and its human health impacts. J Hazard Mater 310:278–279. https://doi.org/10.1016/j.jhazmat.2016.02.043
Nedellec V, Rabl A (2016) Costs of health damage from atmospheric emissions of toxic metals: part 2—analysis for mercury and lead. Risk Anal 36:2096–2104. https://doi.org/10.1111/risa.12598
Salonen JT, Seppänen K, Lakka TA et al (2000) Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 148:265–273. https://doi.org/10.1016/S0021-9150(99)00272-5
Wiggers GA, Furieri LB, Briones AM et al (2016) Cerebrovascular endothelial dysfunction induced by mercury exposure at low concentrations. Neurotoxicology 53:282–289. https://doi.org/10.1016/j.neuro.2016.02.010
Furieri LB, Galán M, Avendaño MS, García-Redondo AB, Aguado A, Martínez S, Cachofeiro V, Bartolomé MV, Alonso MJ, Vassallo DV, Salaices M (2011) Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. Br J Pharmacol 162:1819–1831. https://doi.org/10.1111/j.1476-5381.2011.01203.x
Wiggers GA, Stefanon I, Padilha AS et al (2008) Low nanomolar concentration of mercury chloride increases vascular reactivity to phenylephrine and local angiotensin production in rats. Comp Biochem Physiol - C Toxicol Pharmacol 147:252–260. https://doi.org/10.1016/j.cbpc.2007.10.003
Wiggers GA, Peçanha FM, Briones AM et al (2008) Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. Am J Physiol Heart Circ Physiol 295:H1033–H1043. https://doi.org/10.1152/ajpheart.00430.2008
Vassallo DV, Wiggers GA, Padilha AS, Simões MR (2019) Endothelium:a target for harmful actions of metals. Curr Hypertens Rev 15:1–8. https://doi.org/10.2174/1573402115666190115153759
Rizzetti DA, Torres JGD, Escobar AG et al (2013) Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury. PLoS One 8. https://doi.org/10.1371/journal.pone.0055806
García Gómez M, Boffetta P, Caballero Klink JD et al (2007) Cardiovascular mortality in mercury miners. Med Clin (Barc) 128:766–771
Kobal AB, Horvat M, Prezelj M et al (2004) The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J Trace Elem Med Biol 17:261–274. https://doi.org/10.1016/S0946-672X(04)80028-2
Torres AD, Rai AN, Hardiek ML (2000) Mercury intoxication and arterial hypertension: report of two patients and review of the literature. Pediatrics 105:e34. https://doi.org/10.1542/peds.105.3.e34
Pecanha FM, Wiggers GA, Briones AM, Perez-Giron JV, Miguel M, Garcia-Redondo AB, Vassallo DV, Alonso MJ, Salaices M (2010) The role of cyclooxygenase (COX)-2 derived prostanoids on vasoconstrictor responses to phenylephrine is increased by exposure to low mercury concentration. J Physiol Pharmacol 61:29–36
Clarkson TW (1993) Molecular and ionic mimicry of toxic metals. Annu Rev Phnrmacol Toxicol 32:545–571
Halbach S (1990) Mercury compounds: lipophilicity and toxic effects on isolated myocardial tissue. Arch Toxicol 64:315–319
Abramson JJ, Salama G (1989) Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr 21:283–294. https://doi.org/10.1007/BF00812073
Azevedo BF, Furieri LB, Peçanha FM et al (2012) Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol 2012:1–11. https://doi.org/10.1155/2012/949048
Ward NC, Hodgson JM, Puddey IB et al (2004) Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic Biol Med 36:226–232. https://doi.org/10.1016/j.freeradbiomed.2003.10.021
Lataro RM, Silva MAB, Mestriner FL et al (2019) Chronic treatment with acetylcholinesterase inhibitors attenuates vascular dysfunction in spontaneously hypertensive rats. Am J Hypertens
Lee RM (1985) Vascular changes at the prehypertensive phase in the mesenteric arteries from spontaneously hypertensive rats. Blood Vessels 22:105–126
Puzserova A, Ilovska V, Balis P et al (2014) Age-related alterations in endothelial function of femoral artery in young SHR and WKY rats. Biomed Res Int 2014. https://doi.org/10.1155/2014/658479
Dickhout JG, Lee RM (1998) Blood pressure and heart rate development in young spontaneously hypertensive rats. Am J Phys 274:H794–H800. https://doi.org/10.1038/tp.2017.130
Yamori Y (1994) Development of the spontaneously hypertensive rat (SHR), the stroke-prone SHR (SHRSP) and their various substrain models for hypertension-related cardiovascular disease. Elsevier 16:346–364
Cooper G IV (1987) Cardiocyte adaptation to chronically altered load. Annu Rev Physiol 49:501–518
Gupta M, Bansal JK, Khanna CM (1996) Blood mercury in workers exposed to the preparation of mercury cadmium telluride layers on cadmium telluride base. Ind Health 34:421–425. https://doi.org/10.2486/indhealth.34.421
Chen C, Qu L, Li B, Xing L, Jia G, Wang T, Gao Y, Zhang P, Li M, Chen W, Chai Z (2005) Increased oxidative DNA damage, as assessed byurinary 8-hydroxy-2-deoxyguanosine concentrations, and serum redoxstatus in persons exposed to mercury. Clin Chem 51:759–767
United States Environmental Protection Agency (USEPA) (1998) Mercury health effects. EPA 600/8, Washington
Ibarra M, López-Guerrero JJ, Mejía-Zepeda R, Villalobos-Molina R (2006) Endothelium-dependent inhibition of the contractile response is decreased in aorta from aged and spontaneously hypertensive rats. Arch Med Res 37:334–341. https://doi.org/10.1016/j.arcmed.2005.06.015
Vassallo DV, Simões MR, Giuberti K, Azevedo BF, Ribeiro Junior RF, Salaices M, Stefanon I (2019) Effects of chronic exposure to mercury on angiotensin-converting enzyme activity and oxidative stress in normotensive and hypertensive rats. Arq Bras Cardiol 112:374–380. https://doi.org/10.5935/abc.20180271
Cacanyiova S, Berenyiova A, Kristek F, Drobna M, Ondrias K, Grman M (2016) The adaptive role of nitric oxide and hydrogen Sulphide in vasoactive responses of thoracic aorta is triggered already in young spontaneously hypertensive rats. J Physiol Pharmacol 67:501–512
Radaelli A, Mircoli L, Mori I et al (1998) Nitric oxide-dependent vasodilation in young spontaneously hypertensive rats. Hypertension 32:735–739. https://doi.org/10.1161/01.HYP.32.4.735
Fardin PBA, Simões RP, Schereider IRG, Almenara CCP, Simões MR, Vassallo DV (2019) Chronic mercury exposure in prehypertensive SHRs accelerates hypertension development and activates vasoprotective mechanisms by increasing NO and H2O2 production. Cardiovasc Toxicol:1–14. https://doi.org/10.1007/s12012-019-09545-6
Azevedo BF, Simões MR, Fiorim J et al (2016) Chronic mercury exposure at different concentrations produces opposed vascular responses in rat aorta. Clin Exp Pharmacol Physiol 43:712–719. https://doi.org/10.1111/1440-1681.12578
Ito H, Torii M, Suzuki T (1995) Decreased superoxide dismutase activity and increased superoxide anion production in cardiac hypertrophy of spontaneously hypertensive rats. Clin Exp Hypertens 17:803–816. https://doi.org/10.3109/10641969509033636
Briones AM, Touyz RM (2010) Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 12:135–142. https://doi.org/10.1007/s11906-010-0100-z
Drummond G, Selemidis S, Griendling K, Sobey C (2011) Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10:453–471. https://doi.org/10.1038/nrd3403.Combating
Rizzetti DA, Torres JGD, Escobar AG, da Silva TM, Moraes PZ, Hernanz R, Peçanha FM, Castro MM, Vassallo DV, Salaices M, Alonso MJ, Wiggers GA (2017) The cessation of the long-term exposure to low doses of mercury ameliorates the increase in systolic blood pressure and vascular damage in rats. Environ Res 155:182–192. https://doi.org/10.1016/j.envres.2017.02.022
Kumar KV, Das UN (1993) Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun 19:59–66
Pedro-Botet J, Covas MI, Martín S, Rubiés-Prat J (2000) Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens 14:343–345. https://doi.org/10.1038/sj.jhh.1001034
Pawluk H, Pawluk R, Robaczewska J, Kędziora-Kornatowska K, Kędziora J (2017) Biomarkers of antioxidant status and lipid peroxidation in elderly patients with hypertension. Redox Rep 22:542–546. https://doi.org/10.1080/13510002.2017.1372072
Wang M, Ihida-Stansbury K, Kothapalli D et al (2011) Microsomal prostaglandin E2 synthase-1 modulates the response to vascular injury. Circulation 123:631–639. https://doi.org/10.1161/CIRCULATIONAHA.110.973685
Camacho M, Dilmé J, Solà-Villà D, Rodríguez C, Bellmunt S, Siguero L, Alcolea S, Romero JM, Escudero JR, Martínez-González J, Vila L (2013) Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm. J Lipid Res 54:3506–3515. https://doi.org/10.1194/jlr.m042481
Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Puré E, FitzGerald G (2008) Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation 117:1302–1309. https://doi.org/10.1161/CIRCULATIONAHA.107.731398
Avendaño MS, Martínez-Revelles S, Aguado A et al (2016) Role of COX-2-derived PGE2on vascular stiffness and function in hypertension. Br J Pharmacol 173:1541–1555. https://doi.org/10.1111/bph.13457
Martínez-Revelles S, Avendaño MS, García-Redondo AB et al (2013) Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18:51–65. https://doi.org/10.1089/ars.2011.4335
da Cunha V, Souza HP, Rossoni LV, França AS, Vassallo DV (2000) Effects of mercury on the isolated perfused rat tail vascular bed are endothelium-dependent. Arch Environ Contam Toxicol 39:124–130
Xavier FE, Rossoni LV, Alonso MJ, Balfagón G, Vassallo DV, Salaices M (2004) Ouabain-induced hypertension alters the participation of endothelial factors in α-adrenergic responses differently in rat resistance and conductance mesenteric arteries. Br J Pharmacol 143:215–225. https://doi.org/10.1038/sj.bjp.0705919
Liu D, Liu B, Luo W et al (2015) A vasoconstrictor response to COX-1-mediated prostacyclin synthesis in young rat renal arteries that increases in pre-hypertensive conditions. Am J Physiol Heart Circ Physiol 309:804–811. https://doi.org/10.1152/ajpheart.00150.2015
Funding
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Finance code 001); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Espírito Santo (FAPES—Grant 80600115, 73370215, 80598773). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Ethics Committee of the Federal University of Espirito Santo 09/2018).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simões, R.P., Fardin, P.B.A., Simões, M.R. et al. Long-term Mercury Exposure Accelerates the Development of Hypertension in Prehypertensive Spontaneously Hypertensive Rats Inducing Endothelial Dysfunction: the Role of Oxidative Stress and Cyclooxygenase-2. Biol Trace Elem Res 196, 565–578 (2020). https://doi.org/10.1007/s12011-019-01952-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01952-8