Abstract
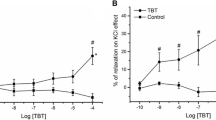
Phthalates are one of the main constituents of plastic, reaching up to 40% of the total plastic weight, and their main function is to impart flexibility/elasticity to polymers that would otherwise be rigid. Phthalates are known as endocrine disruptors, since they can interfere with hormone homeostasis. Regarding the cardiovascular system, it was already shown the effects of di-(2-ethylhexyl) phthalate (DEHP) exposure with significant changes in several calcium-handling proteins and an increase in the blood pressure of mice offspring, suggesting that DEHP leads to vasocontraction. However, the mechanisms involved were not elucidated yet. The aim of this study is to analyse the involvement of calcium channels in the effects induced by DEHP on vascular smooth muscle cells. Endothelium-denuded aorta artery rings were prepared from male Wistar rats and incubated in an organ bath, and the whole-cell configuration of Patch Clamp technique was used to measure the activity of L-type Ca2+ channels (LTCC) in A7r5 cells. Overall, DEHP caused relaxation on KCl-induced contraction at higher concentrations and inhibited the basal and BAY K8644-stimulated calcium current, indicating that this drug blocks LTCC. These results suggest that DEHP induces relaxation on vascular smooth muscle cells due to the inhibition of calcium channels.






Similar content being viewed by others
References
Casals-Casas, C., & Desvergne, B. (2011). Endocrine disruptors: From endocrine to metabolic disruption. Annual Review of Physiology, 73, 135–162.
Wittassek, M., Koch, H. M., Angerer, J., & Bruning, T. (2011). Assessing exposure to phthalates—The human biomonitoring approach. Molecular Nutrition & Food Research, 55, 7–31.
Johns, L. E., Cooper, G. S., Galizia, A., & Meeker, J. D. (2015). Exposure assessment issues in epidemiology studies of phthalates. Environment International, 85, 27–39.
Zota, A. R., Calafat, A. M., & Woodruff, T. J. (2014). Temporal trends in phthalate exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental Health Perspectives, 122, 235–241.
Mariana, M., Feiteiro, J., Verde, I., & Cairrao, E. (2016). The effects of phthalates in the cardiovascular and reproductive systems: A review. Environment International, 94, 758–776.
Posnack, N. G. (2014). The adverse cardiac effects of di(2-ethylhexyl)phthalate and bisphenol A. Cardiovascular Toxicology, 14, 339–357.
Rubin, R. J., & Jaeger, R. J. (1973). Some pharmacologic and toxicologic effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers. Environmental Health Perspectives, 3, 53–59.
Aronson, C. E., Serlick, E. R., & Preti, G. (1978). Effects of di-2-ethylhexyl phthalate on the isolated perfused rat heart. Toxicology and Applied Pharmacology, 44, 155–169.
Gillum, N., Karabekian, Z., Swift, L. M., Brown, R. P., Kay, M. W., & Sarvazyan, N. (2009). Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicology and Applied Pharmacology, 236, 25–38.
Posnack, N. G., Lee, N. H., Brown, R., & Sarvazyan, N. (2011). Gene expression profiling of DEHP-treated cardiomyocytes reveals potential causes of phthalate arrhythmogenicity. Toxicology, 279, 54–64.
Lee, K. I., Chiang, C. W., Lin, H. C., Zhao, J. F., Li, C. T., Shyue, S. K., et al. (2015). Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Archives of Toxicology, 90, 1211–1224.
Vanhoutte, P. M., Shimokawa, H., Tang, E. H., & Feletou, M. (2009). Endothelial dysfunction and vascular disease. Acta Psychologica, 196, 193–222.
Davignon, J., & Ganz, P. (2004). Role of endothelial dysfunction in atherosclerosis. Circulation, 109, III27–32.
Gulati, K., & Lall, S. B. (1996). Angiotensin II—Receptor subtypes characterization and pathophysiological implications. Indian Journal of Experimental Biology, 34, 91–97.
Goldfarb, D. A. (1994). The renin-angiotensin system. New concepts in regulation of blood pressure and renal function. Urologic Clinics of North America, 21, 187–194.
Mariana, M., Feiteiro, J., Cairrao, E., & Verde, I. (2016). Mifepristone is a vasodilator due to the inhibition of smooth muscle cells L-type Ca2+ channels. Reprod Sci, 23, 723–730.
Hamill, O. P., Marty, A., Neher, E., Sakmann, B., & Sigworth, F. J. (1981). Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv European Journal of Physiology, 391, 85–100.
Stull, J. T., Gallagher, P. J., Herring, B. P., & Kamm, K. E. (1991). Vascular smooth muscle contractile elements. Cellular Regulation, Hypertension, 17, 723–732.
Cairrao, E., Alvarez, E., Carvas, J. M., Santos-Silva, A. J., & Verde, I. (2012). Non-genomic vasorelaxant effects of 17beta-estradiol and progesterone in rat aorta are mediated by L-type Ca2+ current inhibition. Acta Pharmacologica Sinica, 33, 615–624.
Alvarez, E., Cairrao, E., Morgado, M., Morais, C., & Verde, I. (2010). Testosterone and cholesterol vasodilation of rat aorta involves L-type calcium channel inhibition. Advances in Pharmacological Sciences, 2010, 534184.
Wang, Y., Hou, R., Li, P., Li, J., Yan, J., Yin, F., et al. (2004). Gene expression profiles in response to the activation of adrenoceptors in A7r5 aortic smooth muscle cells. Clinical and Experimental Pharmacology and Physiology, 31, 602–607.
Guimaraes, S., & Moura, D. (2001). Vascular adrenoceptors: an update. Pharmacological Reviews, 53, 319–356.
Weihua, Z., Saji, S., Makinen, S., Cheng, G., Jensen, E. V., Warner, M., et al. (2000). Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proceedings of the National Academy of Sciences USA, 97, 5936–5941.
Traupe, T., Stettler, C. D., Li, H., Haas, E., Bhattacharya, I., Minotti, R., et al. (2007). Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension, 49, 1364–1370.
Perusquia, M., Hernandez, R., Morales, M. A., Campos, M. G., & Villalon, C. M. (1996). Role of endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aorta. General Pharmacology, 27, 181–185.
vom Saal, F. S., Akingbemi, B. T., Belcher, S. M., Birnbaum, L. S., Crain, D. A., Eriksen, M., et al. (2007). Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive Toxicology, 24, 131–138.
Diamanti-Kandarakis, E., Bourguignon, J. P., Giudice, L. C., Hauser, R., Prins, G. S., Soto, A. M., et al. (2009). Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews, 30, 293–342.
Welshons, W. V., Thayer, K. A., Judy, B. M., Taylor, J. A., & Curran, E. M. (2003). F.S. vom Saal, Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environmental Health Perspectives, 111, 994–1006.
Welshons, W. V., & Nagel, S. C. (2006). F.S. vom Saal, Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology, 147, S56–69.
Bers, D. M. (2008). Calcium cycling and signaling in cardiac myocytes. Annual Review of Physiology, 70, 23–49.
Posnack, N. G., Idrees, R., Ding, H., Jaimes, R., 3rd, Stybayeva, G., Karabekian, Z., et al. (2015). Exposure to phthalates affects calcium handling and intercellular connectivity of human stem cell-derived cardiomyocytes. PLoS ONE, 10, e0121927.
Philips, E. M., Jaddoe, V. W., & Trasande, L. (2017). Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reproduction Toxicology, 68, 105–118.
Parks, L. G., Ostby, J. S., Lambright, C. R., Abbott, B. D., Klinefelter, G. R., Barlow, N. J., et al. (2000). The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences, 58, 339–349.
Howdeshell, K. L., Wilson, V. S., Furr, J., Lambright, C. R., Rider, C. V., Blystone, C. R., et al. (2008). A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicological Sciences, 105, 153–165.
Wilson, V. S., Lambright, C., Furr, J., Ostby, J., Wood, C., Held, G., et al. (2004). Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicology Letters, 146, 207–215.
Talsness, C. E., Andrade, A. J., Kuriyama, S. N., Taylor, J. A., & vom Saal, F. S. (2009). Components of plastic: Experimental studies in animals and relevance for human health. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 364, 2079–2096.
Mankidy, R., Wiseman, S., Ma, H., & Giesy, J. P. (2013). Biological impact of phthalates. Toxicology Letters, 217, 50–58.
Andrade, A. J., Grande, S. W., Talsness, C. E., Grote, K., & Chahoud, I. (2006). A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicology, 227, 185–192.
Acknowledgements
This work is supported by FEDER funds through the POCI—COMPETE 2020—Operational Programme Competitiveness and Internationalisation in Axis I—Strengthening research, technological development and innovation (Project POCI-01-0145-FEDER-007491) and National Funds by FCT—Foundation for Science and Technology (Project UID/Multi/00709/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Mariana, M., Feiteiro, J. & Cairrao, E. Cardiovascular Response of Rat Aorta to Di-(2-ethylhexyl) Phthalate (DEHP) Exposure. Cardiovasc Toxicol 18, 356–364 (2018). https://doi.org/10.1007/s12012-017-9439-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9439-6