Abstract
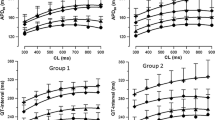
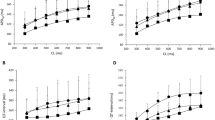
The potential of ondansetron and domperidone, both clinically established antiemetic agents, to increase the QT-interval has been described in several case reports. Therefore, the aim of the present study was to investigate whether these drugs may provoke polymorphic ventricular tachycardia in a sensitive experimental model of drug-induced proarrhythmia. In 10 female rabbits, ondansetron (1, 5 and 10 µM, n = 10) or domperidone (0.5, 1 and 2 µM, n = 8) was infused after obtaining baseline data. Eight endo- and epicardial monophasic action potentials and a simultaneously recorded 12-lead ECG reproduced the clinically observed QT-prolongation (ondansetron: 1 µM:+17 ms, 5 µM:+41 ms, 10 µM:+78 ms, p < 0.01; domperidone: 0.5 µM:+57 ms, 1 µM:+79 ms, 2 µM:+99 ms, p < 0.01). This was accompanied by a significant increase in action potential duration at 90% of repolarization. Administration of both agents also increased dispersion of repolarization (ondansetron: 1 µM:+12 ms, 5 µM:+17 ms; 10 µM:+18 ms, p < 0.05; domperidone: 0.5 µM:+19 ms, 1 µM:+27 ms; 2 µM:+23 ms p < 0.05). Lowering of potassium concentration in bradycardic AV-blocked hearts provoked early afterdepolarizations (EADs) in 9 of 10 ondansetron-treated hearts and induced polymorphic ventricular tachycardia (VT) resembling torsade de pointes in 7 of 10 ondansetron-treated hearts (86 episodes). Under the influence of domperidone, EAD and polymorphic VT occurred in 7 of 8 hearts (131 episodes). In the present study, both ondansetron and domperidone demonstrated a severe proarrhythmic potential. A significant prolongation of cardiac repolarization as well as a marked increase in spatial dispersion of repolarization represents the underlying electrophysiologic mechanisms. These results imply that application of ondansetron should be handled carefully. For regular administration, ECG monitoring should be mandatory.





Similar content being viewed by others
References
Charbit, B., Albaladejo, P., Funck-Brentano, C., Legrand, M., Samain, E., & Marty, J. (2005). Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology, 102, 1094–1100.
Schnell, F. M., & Coop, A. J. (2005). An evaluation of potential signals for ventricular arrhythmia and cardiac arrest with dolasetron, ondansetron, and granisetron in the FDA combined spontaneous reporting system/adverse event reporting system. Current Therapeutic Research, Clinical and Experimental, 66, 409–419.
Hafermann, M. J., Namdar, R., Seibold, G. E., & Page, R. L. (2011). Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: A prospective, observational study. Drug, Healthcare and Patient Safety, 3, 53–58.
McKechnie, K., & Froese, A. (2010). Ventricular tachycardia after ondansetron administration in a child with undiagnosed long QT syndrome. Canadian Journal of Anaesthesia, 57, 453–457.
Claassen, S., & Zunkler, B. J. (2005). Comparison of the effects of metoclopramide and domperidone on HERG channels. Pharmacology, 74, 31–36.
Rocha, C. M., & Barbosa, M. M. (2005). QT interval prolongation associated with the oral use of domperidone in an infant. Pediatric Cardiology, 26, 720–723.
Boyce, M. J., Baisley, K. J., & Warrington, S. J. (2012). Pharmacokinetic interaction between domperidone and ketoconazole leads to QT prolongation in healthy volunteers: A randomized, placebo-controlled, double-blind, crossover study. British Journal of Clinical Pharmacology, 73, 411–421.
Doggrell, S. A., & Hancox, J. C. (2014). Cardiac safety concerns for domperidone, an antiemetic and prokinetic, and galactogogue medicine. Expert Opinion on Drug Safety, 13, 131–138.
Biewenga, J., Keung, C., Solanki, B., Natarajan, J., Leitz, G., Deleu, S., et al. (2015). Absence of QTc prolongation with domperidone: A randomized, double-blind, placebo- and positive-controlled thorough QT/QTc study in healthy volunteers. Clinical Pharmacology in Drug Development, 4, 41–48.
Frommeyer, G., Milberg, P., Witte, P., Stypmann, J., Koopmann, M., Lucke, M., et al. (2011). A new mechanism preventing proarrhythmia in chronic heart failure: Rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure. European Journal of Heart Failure, 13, 1060–1069.
Frommeyer, G., Fischer, C., Lange, P. S., Leitz, P., Fehr, M., Bogossian, H., et al. (2016). Divergent electrophysiologic profile of fluconazole and voriconazole in an experimental whole-heart model of proarrhythmia. European Journal of Pharmacology, 776, 185–190.
Frommeyer, G., Brucher, B., Von der Ahe, H., Kaese, S., Dechering, D. G., Kochhauser, S., et al. (2016). Low proarrhythmic potential of citalopram and escitalopram in contrast to haloperidol in an experimental whole-heart model. European Journal of Pharmacology, 788, 192–199.
Milberg, P., Eckardt, L., Bruns, H. J., Biertz, J., Ramtin, S., Reinsch, N., et al. (2002). Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: Fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. Journal of Pharmacology and Experimental Therapeutics, 303, 218–225.
Eckardt, L., Breithardt, G., & Haverkamp, W. (2002). Electrophysiologic characterization of the antipsychotic drug sertindole in a rabbit heart model of torsade de pointes: Low torsadogenic potential despite QT prolongation. Journal of Pharmacology and Experimental Therapeutics, 300, 64–71.
Roden, D. M. (1998). Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing and Clinical Electrophysiology: PACE, 21, 1029–1034.
Hondeghem, L. M., & Snyders, D. J. (1990). Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation, 81, 686–690.
Antzelevitch, C. (2008). Drug-induced spatial dispersion of repolarization. Cardiology Journal, 15, 100–121.
Verduyn, S. C., Vos, M. A., Van der Zande, J., Kulcsar, A., & Wellens, H. J. (1997). Further observations to elucidate the role of interventricular dispersion of repolarization and early afterdepolarizations in the genesis of acquired torsade de pointes arrhythmias: A comparison between almokalant and d-sotalol using the dog as its own control. Journal of the American College of Cardiology, 30, 1575–1584.
Eckardt, L., Haverkamp, W., Borggrefe, M., & Breithardt, G. (1998). Experimental models of torsade de pointes. Cardiovascular Research, 39, 178–193.
Sardi, I., La Marca, G., Cardellicchio, S., Giunti, L., Malvagia, S., Genitori, L., et al. (2013). Pharmacological modulation of blood-brain barrier increases permeability of doxorubicin into the rat brain. American Journal of Cancer Research, 3, 424–432.
Drolet, B., Rousseau, G., Daleau, P., Cardinal, R., & Turgeon, J. (2000). Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation, 102, 1883–1885.
Acknowledgements
This study was supported by the German Cardiac Society (to G.F.) and the Dr. Peter Osypka Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Frommeyer, G., Fischer, C., Ellermann, C. et al. Severe Proarrhythmic Potential of the Antiemetic Agents Ondansetron and Domperidone. Cardiovasc Toxicol 17, 451–457 (2017). https://doi.org/10.1007/s12012-017-9403-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9403-5