Abstract
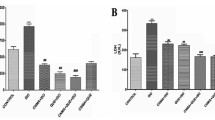
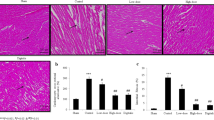
Combined effects of vincristine and quercetin in the regulation of isoproterenol (ISO)-induced cardiac necrosis have been evaluated in rats. ISO administration (100 mg/kg, s.c., for two consecutive days) increased the levels of serum creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), glutamate pyruvate transaminase (SGPT) and cardiac troponin (cTnT) as well as cardiac lipid peroxidation products (malondialdehyde and lipid hydroperoxides). However, it reduced the activities of superoxide dismutase (SOD), catalase and the glutathione peroxidase and the level of reduced glutathione. It also increased the heart rate and ST-segment elevation in ECG. Pretreatment of vincristine (25 μg/kg) or quercetin (10 mg/kg) alone for 2 weeks ameliorated these cardiotoxic effects partially. However, treatment of both vincristine and quercetin for a similar period reduced the serum CK-MB, LDH, SGPT and cTnT levels near to normal levels in ISO-treated rats. Concomitantly, the test drugs improved the status of antioxidants and decreased the cardiac lipid peroxidation products. Combined treatment of both the drugs also restored the pathological electrocardiographic patterns and reduced the area of myocardial necrosis. Histopathology of heart in ISO-administered rats that received both vincristine and quercetin showed nearly normal myocardium with very little inflammatory infiltration. In conclusion, the present finding appears to be the first one, suggesting a better protection of cardiac tissues by combined treatment of vincristine and quercetin in isoproterenol-induced cardiac toxicity.





Similar content being viewed by others
References
Rona, G. (1985). Catecholamine cardiotoxicity. Journal of Molecular and Cellular Cardiology, 17, 291–306.
Wexler, B. C., & Greenberg, B. P. (1978). Protective effects of clofibrate on isoproterenol-induced myocardial infarction in arteriosclerotic and nonarteriosclerotic rats. Atherosclerosis, 29, 373–395.
Jay, L. Z., & Hassan, M. A. (2006). The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research, 70, 181–190.
Thompson, J. A., & Hess, M. L. (1986). The oxygen free radical system: a fundamental mechanism in the production of myocardial necrosis. Progress in Cardiovascular Diseases, 28, 449–462.
Chatterjee, K., Zhang, J., Tao, R., Honbo, N., & Karliner, J. S. (2008). Vincristine attenuates doxorubicin cardiotoxicity. Biochemical and Biophysical Research Communications, 373, 555–560.
Jordon, M. A. (2002). Anti-cancer agents. Current Medicinal Chemistry, 2, 1–17.
Tochinai, R., Ando, M., Suzuki, T., Suzuki, K., Nagata, Y., Hata, C., et al. (2013). Histopathological studies of microtubule disassembling agent-induced myocardial lesions in rats. Experimental and Toxicologic Pathology, 65, 737–743.
Xuan, D. N., Paul, L. R., Thomas, N., Harald, K., & Dieter, B. (2011). Rapid treatment of leukostasis in leukemic mantle cell lymphoma using therapeutic leukapheresis: A case report. The Scientific World Journal, 11, 1554–1559.
Panda, S., Kar, A., & Ramamurthy, V. (2013). Cardioprotective effect of vincristine on isoproterenol-induced myocardial necrosis in rats. European Journal of Pharmacology, 723, 451–458.
Perez-Vizcaino, F., & Duarte, J. (2010). Flavonols and cardiovascular disease. Molecular Aspects of Medicine, 31, 478–944.
Benavente-Garcıa, O., & Castillo, J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. Journal of Agriculture and Food Chemistry, 56, 6185–6205.
Sestili, P., Guidarelli, A., Dacha, M., & Cantoni, O. (1998). Quercetin prevents DNA single strand breakage and cytotoxicity caused by tert-butylhydroperoxide: Free radical scavenging versus iron chelating mechanism. Free Radical Biology & Medicine, 25, 196–200.
Panda, S., Kar, A., Banerjee, T., & Sharma, N. (2012). Combined effects of quercetin and atenolol in reducing isoproterenol-induced cardiotoxicity in rats: possible mediation through scavenging free radicals. Cardiovascular Toxicology, 12, 235–242.
Lu, G. P., Schwalbe, S. S., Marx, G. F., Batiller, G., & Limjoco, R. (1992). Hypoglycemia enhances bupivacaine-induced cardiotoxicity in the rat. Journal of Anesthesia, 6, 255–261.
Panda, S., Kar, A., Sharma, P., & Sharma, A. (2013). Cardioprotective potential of N, α-l-rhamnopyranosyl vincosamide, an indole alkaloid isolated from the leaves of Moringa olefera in isoproterenol-induced cardiotoxic rats: An in vivo and in vitro study. Bioorganic & Medicinal Chemistry Letters, 23, 959–962.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assays of lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Jiang, Z. Y., Hunt, J. V., & Wolff, S. P. (1992). Detection of lipid hydroperoxides using the ‘fox method’. Analytical Biochemistry, 202, 384–389.
Marklund, S., & Marklund, G. (1974). Involvement of superoxide anion radical in the autoxidation of pyrogallol: A convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469–474.
Aebi, H. E. (1983). Catalase. In H. U. Bergmeyer (Ed.), Methods in enzymatic analysis (Vol. 2, pp. 276–286). New York: Academic Press.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70–77.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Lie, J. T., Pairolero, P. C., Holley, K. E., & Titus, J. L. (1975). Marcroscopic enzyme mapping verification of large, homogenous, experimental myocardial infarcts of predictable size and location in dogs. The Journal of Thoracic and Cardiovascular Surgery, 69, 599–605.
Judd, J. T., & Wexler, B. C. (1974). Myocardial glycoprotein changes with isoproterenol induced necrosis and repair in the rat. American Journal of Physiology, 226, 597–602.
Kannan, M. M., & Quine, S. D. (2011). Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. European Journal of Pharmacology, 659, 45–52.
Priscilla, D. H., & Prince, P. S. (2009). Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chemico-Biological Interactions, 179, 118–124.
Prince, P. M. S., & Sathya, B. (2010). Pretreatment with quercetin ameliorates lipids, lipoproteins and marker enzymes of lipid metabolism in isoproterenol treated cardiotoxic male Wistar rats. European Journal of Pharmacology, 635, 142–148.
Patel, V., Upaganlawar, A., Zalawadia, R., & Balaraman, R. (2010). Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. European Journal of Pharmacology, 644, 160–168.
Bahit, M. C., Criger, D. A., Ohman, E. M., Granger, C. B., & Wagner, G. S. (2002). Thresholds for the electrocardiographic change range of biochemical markers of acute myocardial infarction. American Journal of Cardiology, 90, 233–237.
Ahmed, L. A., Salem, H. A., Attia, A. S., & EI-Sayed, M. E. (2009). Enhancement of amlodipine cardioprotection by quercetin in ischemia/reperfusion injury in rats. Pharmacy and Pharmacology, 61, 1233–1241.
Blasig, I. E., Blasig, R., & Lowe, H. (1984). Myocardial lipid peroxidation during isoproterenol induced blood flow reduction in rat myocardium. Biomedica Biochimica Acta, 43, 171–174.
Wattanapitayakul, S. K., & Bauer, J. A. (2001). Oxidative pathways in cardiovascular disease roles, mechanisms, and therapeutic implications. Pharmacology & Therapeutics, 89, 187–206.
Wu, J., Hecker, J. G., & Chiamvimonvat, N. (2009). Antioxidant enzyme gene transfer for ischemic diseases. Advanced Drug Delivery Reviews, 61, 351–363.
Rajadurai, M., & Stanely Mainzen Prince, P. (2006). Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardio toxicity in Wistar rats: biochemical and histopathological evidences. Toxicology, 228, 259–268.
Kandaswamy, S. K., & Prince, P. S. M. (2010). Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress and Chaperones, 15, 791–806.
Hamberg, M., Svensson, J., Wakabayashi, T., & Samuelsson, B. (1974). Isolation and structure of two prostaglandins endoperoxides that cause platelet aggregation. In Proceedings of the national academy of sciences (vol 71, 345e349).
Todd, G. L., Cullan, G. E., & Cullan, G. M. (1980). Isoproterenol induced myocardial necrosis and membrane permeability alterations in the isolated perfused rabbit heart. Experimental and Molecular Pathology, 3, 43–54.
Prabhu, S., Mallika, J., Sabitha, K. E., & Shyamala Devi, C. S. (2006). Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. Journal of Ethnopharmacology, 107, 126–133.
Zhang, J., Chatterjee, K., Conrad, C., Alano, M., Norman, H., & Joel, S. K. (2010). Vincristine Attenuates N-methyl-N′-nitro-N-nitrosoguanidine-induced poly-(ADP) ribose polymerase activity in cardiomyocytes. Journal of Cardiovascular Pharmacology, 55, 219–226.
Uchiyama, T., Englemanm, R. M., Maulik, N., & Das, D. K. (2004). Role of AKTsignaling in mitochondrial survival pathways triggered by hypoxic preconditioning. Circulation, 109, 3042–3049.
Chow, J. M., Shen, S. C., Huan, S. K., Lin, H. Y., & Chen, Y. C. (2005). Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochemical Pharmacology, 69, 1839e1851.
Johnson, J. L., Rupasinghe, S. G., Stefani, F., Schuler, M. A., & de Gonzalez, M. E. (2011). Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. Journal of Medicinal Food, 14, 325–333.
Acknowledgments
This work was supported by the grant received from Department of Science and Technology (DST), under Women Scientist scheme to Dr. S. Panda [REF: SR/WOS-A/LS-259], New Delhi, India.
Conflict of interest
Authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, S., Kar, A. Combined Effects of Vincristine and Quercetin in Reducing Isoproterenol-Induced Cardiac Necrosis in Rats. Cardiovasc Toxicol 15, 291–299 (2015). https://doi.org/10.1007/s12012-014-9291-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-014-9291-x