Abstract
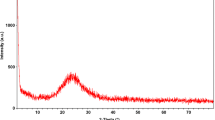
The present study aimed to determine the cytotoxicity of chromium(III) oxide micro particles (Cr2O3-Ps) in rainbow trout (Oncorhynchus mykiss) spermatozoa. Firstly, Cr2O3-Ps were synthesized and structurally characterized the surface, morphological for particle size and thermal properties. In addition, its structural and elemental purity was determined using energy-dispersive X-ray (EDX) spectrum and elemental maps. Structural purity, thermal properties, and stability of Cr2O3-Ps were also examined in detail by performing thermal analysis techniques. The cytotoxicity of Cr2O3-Ps was measured by the observation of velocities, antioxidant activities, and DNA damages in rainbow trout spermatozoa after exposure during 3 h in vitro incubation. The straight line velocity (VSL), the curvilinear velocity (VCL), and the angular path velocity (VAP) of spermatozoa decreased after exposure to Cr2O3-Ps. While the superoxide dismutase (SOD) and the catalase (CAT) decreased, the lipid peroxidation increased in a dose-dependent manner. However, the total glutathione (tGSH) was not affected in this period. DNA damages were also determined in spermatozoa using Comet assay. According to DNA in tail (%) data, DNA damages have been detected with gradually increasing concentrations of Cr2O3-Ps. Furthermore, all of class types which are categorized as the intensity of DNA fragmentation has been observed between 50 and 500 µg/L concentrations of Cr2O3-Ps exposed to rainbow trout spermatozoa. At the end of this study, we determined that the effective concentrations (EC50) were 76.67 µg/L for VSL and 87.77 µg/L for VCL. Finally, these results about Cr2O3-Ps may say to be major risk concentrations over 70 µg/L for fish reproduction in aquatic environments.








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current research are available from the corresponding author on reasonable request.
References
Zhu Y, Liu X, Hu Y et al (2019) Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ Res 174:54–60
Du J, Xu S, Zhou Q et al (2019) The ecotoxicology of titanium dioxide nanoparticles, an important engineering nanomaterial. Toxicol Environ Chem 101:165–189
Chavali MS, Nikolova MP (2019) Metal oxide nanoparticles and their applications in nanotechnology. SN Appl Sci 1:. https://doi.org/10.1007/s42452-019-0592-3
Mohanapandian K, Krishnan A (2014) Effect of concentration of Ni2+ on the physio chemical properties of Cr2o3 nano particles. Adv Stud Theor Phys. https://doi.org/10.12988/astp.2014.312139
Ivanova T, Gesheva K, Cziraki A, et al (2008) Structural transformations and their relation to the optoelectronic properties of chromium oxide thin films. J Phys Conf Ser 113:. https://doi.org/10.1088/1742-6596/113/1/012030
Alarifi S, Ali D, Alkahtani S (2016) Mechanistic investigation of toxicity of chromium oxide nanoparticles in murine fibrosarcoma cells. Int J Nanomedicine 11:. https://doi.org/10.2147/IJN.S99995
khorsandi K, Rabbani-Chadegani A (2013) Studies on the genotoxic effect of chromium oxide (Cr VI): Interaction with deoxyribonucleic acid in solution. Mutat Res - Genet Toxicol Environ Mutagen 750:. https://doi.org/10.1016/j.mrgentox.2012.10.002
Maitlo HA, Kim KH, Kumar V, et al (2019) Nanomaterials-based treatment options for chromium in aqueous environments. Environ. Int. 130
Canadian Water Quality Guidelines (1999) Canadian water quality guidelines for the protection of aquatic life. Can Counc Minist Environ 1–5
EPA (2015) Water: chromium in drinking water. In: United States Environmwntal Prot. Agency
He X, Aker WG, Fu PP, Hwang HM (2015) Toxicity of engineered metal oxide nanomaterials mediated by nano-bio-eco-interactions: a review and perspective. Environ Sci Nano 2:564–582
U.S Geological Survey (2020) Mineral Commodity Summaries 2020
Puerari RC, da Costa CH, Vicentini DS et al (2016) Synthesis, characterization and toxicological evaluation of Cr2O3 nanoparticles using Daphnia magna and Aliivibrio fischeri. Ecotoxicol Environ Saf 128:36–43. https://doi.org/10.1016/j.ecoenv.2016.02.011
Chusuei CC, Wu CH, Mallavarapu S et al (2013) Cytotoxicity in the age of nano: the role of fourth period transition metal oxide nanoparticle physicochemical properties. Chem Biol Interact 206:319–326. https://doi.org/10.1016/j.cbi.2013.09.020
Cho WS, Duffin R, Bradley M, et al (2013) Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part FibreToxicol 10:. https://doi.org/10.1186/1743-8977-10-55
Wang X, Zheng Q, Yuan Y et al (2017) Bacterial community and molecular ecological network in response to Cr2O3 nanoparticles in activated sludge system. Chemosphere 188:10–17. https://doi.org/10.1016/j.chemosphere.2017.08.072
da Costa CH, Perreault F, Oukarroum A et al (2015) Effect of chromium oxide (III) nanoparticles on the production of reactive oxygen species and photosystem II activity in the green alga Chlamydomonas reinhardtii. Sci Total Environ 565:951–960. https://doi.org/10.1016/j.scitotenv.2016.01.028
Tavares KP, Caloto-Oliveira Á, Vicentini DS et al (2014) Acute toxicity of copper and chromium oxide nanoparticles to Daphnia similis. Ecotoxicol Environ Contam 9:43–50. https://doi.org/10.5132/eec.2014.01.006
Kaweeteerawat C, Ivask A, Liu R et al (2015) Toxicity of metal oxide nanoparticles in Escherichia coli correlates with conduction band and hydration energies. Environ Sci Technol 49:1105–1112. https://doi.org/10.1021/es504259s
Lebedev S, Gavrish I, Rusakova E et al (2018) Influence of various chromium compounds on physiological, morpho-biochemical parameters, and digestive enzymes activity in Wistar rats. Trace Elem Electrolytes 35:242–245. https://doi.org/10.5414/tex0155419
Li J, Song Y, Wu K et al (2018) Effects of Cr2O3 nanoparticles on the chlorophyll fluorescence and chloroplast ultrastructure of soybean (Glycine max). Environ Sci Pollut Res 25:19446–19457. https://doi.org/10.1007/s11356-018-2132-x
Özgür ME, Ulu A, Özcan İ et al (2019) Investigation of toxic effects of amorphous SiO2 nanoparticles on motility and oxidative stress markers in rainbow trout sperm cells. Environ Sci Pollut Res 26:15641–15652. https://doi.org/10.1007/s11356-019-04941-5
Galwey AK, Pöppl L, Rajam S (1983) A melt mechanism for the thermal decomposition of ammonium dichromate. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 79:2143–2151. https://doi.org/10.1039/F19837902143
Mahieu B, Apers DJ, Capron PC (1971) Thermal decomposition of ammonium dichromate. J Inorg Nucl Chem 33:2857–2866. https://doi.org/10.1016/0022-1902(71)80047-7
Lima MD, Bonadimann R, de Andrade MJ, et al (2006) Nanocrystalline Cr2O3 and amorphous CrO3 produced by solution combustion synthesis. J Eur Ceram Soc 26:.https://doi.org/10.1016/j.jeurceramsoc.2005.01.042
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Aebi H (1984) Catalase in vitro. In: Methods in Enzymology. pp 121–126
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055. https://doi.org/10.1016/0003-2697(69)90079-7
Akerboom TPM, Sies H (1981) Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–382. https://doi.org/10.1016/S0076-6879(81)77050-2
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:. https://doi.org/10.1016/S0076-6879(78)52032-6
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Sone BT, Manikandan E, Gurib-Fakim A, Maaza M (2016) Single-phase α-Cr2O3nanoparticles’ green synthesis using Callistemon viminalis’ red flower extract. Green Chem Lett Rev 9:85–90. https://doi.org/10.1080/17518253.2016.1151083
Abdullah MM, Rajab FM, Al-Abbas SM (2014) Structural and optical characterization of Cr2O3 nanostructures: evaluation of its dielectric properties. AIP Adv 4:.https://doi.org/10.1063/1.4867012
Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez MP (2010) Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction 139:989–997. https://doi.org/10.1530/REP-10-0037
Agrawal S, Kango N (2019) Development and catalytic characterization of L-asparaginase nano-bioconjugates. Int J Biol Macromol 135:1142–1150. https://doi.org/10.1016/j.ijbiomac.2019.05.154
Özgür ME, Balcıoğlu S, Ulu A et al (2018) The in vitro toxicity analysis of titanium dioxide (TiO2) nanoparticles on kinematics and biochemical quality of rainbow trout sperm cells. Environ Toxicol Pharmacol 62:11–19. https://doi.org/10.1016/j.etap.2018.06.002
Préaubert L, Tassistro V, Auffan M et al (2018) Very low concentration of cerium dioxide nanoparticles induce DNA damage, but no loss of vitality, in human spermatozoa. Toxicol Vitr 50:236–241. https://doi.org/10.1016/j.tiv.2018.03.013
Nikolovski D, Cumic J, Pantic I (2019) Application of gray level co-occurrence matrix algorithm for detection of discrete structural changes in cell nuclei after exposure to iron oxide nanoparticles and 6-hydroxydopamine. Microsc Microanal 25:982–988. https://doi.org/10.1017/S1431927619014594
Santonastaso M, Mottola F, Colacurci N et al (2019) In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol Reprod Dev 86:1369–1377. https://doi.org/10.1002/mrd.23134
Barkhade T, Mahapatra SK, Banerjee I (2019) Study of mitochondrial swelling, membrane fluidity and ROS production induced by nano-TiO2 and prevented by Fe incorporation. Toxicol Res (Camb) 8:711–722. https://doi.org/10.1039/c9tx00143c
Afifi M, Saddick S, Abu Zinada OA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23:754–760. https://doi.org/10.1016/j.sjbs.2016.06.008
Özgür ME, Ulu A, Balcioğlu S et al (2018) The toxicity assessment of iron oxide (Fe 3 O 4) nanoparticles on physical and biochemical quality of rainbow trout spermatozoon. Toxics 6:62. https://doi.org/10.3390/toxics6040062
Sinha S, Saxena R, Singh S (2005) Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: role of antioxidants and antioxidant enzymes. Chemosphere 58:595–604. https://doi.org/10.1016/j.chemosphere.2004.08.071
Adebayo OA, Akinloye O, Adaramoye OA (2018) Cerium oxide nanoparticle elicits oxidative stress, endocrine imbalance and lowers sperm characteristics in testes of balb/c mice. Andrologia 50:e12920. https://doi.org/10.1111/and.12920
Dietrich GJ, Szpyrka A, Wojtczak M et al (2005) Effects of UV irradiation and hydrogen peroxide on DNA fragmentation, motility and fertilizing ability of rainbow trout (Oncorhynchus mykiss) spermatozoa. Theriogenology 64:1809–1822. https://doi.org/10.1016/j.theriogenology.2005.04.010
Aitken RJ, Koopman P, Lewis SEM (2004) Seeds of concern. Nature 432:48–52
Labbe C, Maisse G, Müller K et al (1995) Thermal acclimation and dietary lipids alter the composition, but not fluidity, of trout sperm plasma membrane. Lipids 30:23–33. https://doi.org/10.1007/BF02537038
Li ZH, Li P, Dzyuba B, Randak T (2010) Influence of environmental related concentrations of heavy metals on motility parameters and antioxidant responses in sturgeon sperm. Chem Biol Interact 188:473–477. https://doi.org/10.1016/j.cbi.2010.09.005
Li P, Li ZH, Dzyuba B et al (2010) Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio, L.) sperm caused by cryopreservation techniques. Biol Reprod 83:852–858. https://doi.org/10.1095/biolreprod.110.085852
Linhartova P, Gazo I, Shaliutina A, Hulak M (2013) The in vitro effect of duroquinone on functional competence, genomic integrity, and oxidative stress indices of sterlet (Acipenser ruthenus) spermatozoa. Toxicol Vitr 27:1612–1619. https://doi.org/10.1016/j.tiv.2013.04.002
Cabrita E, Sarasquete C, Martínez-Páramo S et al (2010) Cryopreservation of fish sperm: applications and perspectives. J Appl Ichthyol 26:623–635
Özgür ME, Okumuş FK (2019) A novel computer assisted sperm analyzer for assessment of spermatozoa motility in fish; BASA-Sperm Aqua. El-Cezeri Fen ve Mühendislik Derg. https://doi.org/10.31202/ecjse.486342
Acknowledgements
Analyses for study were supported by physicochemical and biochemical laboratories in Inönü University and Malatya Turgut Özal University. Therefore, the authors are grateful to the universities for providing the resources to develop this study.
Author information
Authors and Affiliations
Contributions
Mustafa Erkan Özgür: supervision, review, investigation, methodology, conceptualization, formal analysis, writing, review and editing. Ahmet Ulu: investigation, methodology, formal analysis, writing and editing. Canbolat Gürses: investigation, methodology, formal analysis, review and editing. İmren Özcan: formal analysis. Samir Abbas Ali Noma: formal analysis. Süleyman Köytepe: supervision, methodology, validation, writing, review and editing. Burhan Ateş: supervision, methodology, review and editing, conceptualization.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable. The local ethics committee was notified and committee approval was not necessary.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özgür, M.E., Ulu, A., Gürses, C. et al. The Cytotoxicity, DNA Fragmentation, and Decreasing Velocity Induced By Chromium(III) Oxide on Rainbow Trout Spermatozoa. Biol Trace Elem Res 201, 968–983 (2023). https://doi.org/10.1007/s12011-022-03211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03211-9