Abstract
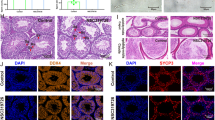
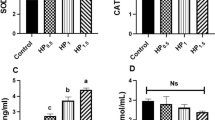
Lead (Pb), a toxic pollutant, is toxic to the testis. However, biological events during testicular Pb poisoning were not well understood. Selenium (Se) has the ability to antagonize Pb toxicity. The purpose of this research was to clarify the relief mechanism of Se on testicular toxicity of Pb from the perspective of oxidative stress, inflammation, heat shock response, and autophagy in a chicken model. Sixty male Hyline chickens (7-day-old) were randomly assigned into four groups. The feeding program consisted of a commercial diet, a Se-supplemented diet (1 mg kg−1 Se), a Pb-supplemented diet (350 mg L−1 Pb), and a Se- and Pb-supplemented diet, respectively. On the 12th week, serums were collected to measure testosterone level and testes were removed to determine testis weight, histological structure, Pb and Se concentrations, oxidative stress indicators, and mRNA and protein expression of inflammatory cytokines, heat shock proteins, and autophagy-related genes. The results showed that Pb poisoning changed the histological structure of testes; decreased serum testosterone level, testis weight, catalase, glutathione-s-transferase, and total antioxidative capacity activities; increased hydrogen peroxide content; inhibited interleukin (IL)-2 and mammalian target of rapamycin expression; and promoted IL-4, IL-12β, heat shock proteins, Beclin 1, Dynein, autophagy-related proteins 5, light chain 3 (LC3)-I, and LC3-II expression in the testes of chickens. Se intervention mitigated the aforementioned alterations induced by Pb. In conclusion, Pb led to oxidative stress, which triggered inflammation, heat shock response, and autophagy. Se administration mitigated testicular toxicity of Pb mainly by mitigating oxidative stress in male chickens.








Similar content being viewed by others
References
Karrari P, Mehrpour O, Abdollahi M (2012) A systematic review on status of lead pollution and toxicity in Iran; guidance for preventive measures. Daru 20. doi:Artn 2 https://doi.org/10.1186/1560-8115-20-2
Yabe J, Nakayama SM, Nakata H, Toyomaki H, Yohannes YB, Muzandu K, Kataba A, Zyambo G, Hiwatari M, Narita D, Yamada D, Hangoma P, Munyinda NS, Mufune T, Ikenaka Y, Choongo K, Ishizuka M (2020) Current trends of blood lead levels, distribution patterns and exposure variations among household members in Kabwe, Zambia. Chemosphere 243:125412. https://doi.org/10.1016/j.chemosphere.2019.125412
Huang H, An Y, Jiao W, Wang J, Li S, Teng X (2018) CHOP/caspase-3 signal pathway involves in mitigative effect of selenium on lead-induced apoptosis via endoplasmic reticulum pathway in chicken testes. Environ Sci Pollut Res Int 25:18838–18845. https://doi.org/10.1007/s11356-018-1950-1
Yang QZ, Liu XR, Chen J, Wen Y, Liu H, Peng ZJ, Yeerken R, Wang LR, Li XH (2020) Lead-mediated inhibition of lysine acetylation and succinylation causes reproductive injury of the mouse testis during development. Toxicol Lett 318:30–43. https://doi.org/10.1016/j.toxlet.2019.10.012
Vallverdu-Coll N, Mougeot F, Ortiz-Santaliestra ME, Castano C, Santiago-Moreno J, Mateo R (2016) Effects of lead exposure on sperm quality and reproductive success in an avian model. Environ Sci Technol 50(22):12484–12492. https://doi.org/10.1021/acs.est.6b04231
Eren H, Mercantepe T, Tumkaya L, Mercantepe F, Dil E, Horsanali MO, Yilmaz A (2020) Evaluation of the protective effects of amifostine and melatonin against cisplatin induced testis injury via oxidative stress and apoptosis in rats. Exp Mol Pathol 112:104324. https://doi.org/10.1016/j.yexmp.2019.104324
Jiao WY, Han Q, Xu YM, Jiang HJ, Xing HJ, Teng XH (2019) Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: through oxidative stress and apoptosis. Fish Shellfish Immunol 86:239–245. https://doi.org/10.1016/j.fsi.2018.08.060
Tenorio MB, Ferreira RC, Moura FA, Bueno NB, de Oliveira MCM, Goulart MOF (2019) Cross-talk between oxidative stress and inflammation in preeclampsia. Oxid Med Cell Longev 2019. doi:Artn 8238727 https://doi.org/10.1155/2019/8238727
Qianru C, Xueyuan H, Bing Z, Qing Z, Kaixin Z, Shu L (2021) Regulation of H2S-induced necroptosis and inflammation in broiler bursa of Fabricius by the miR-15b-5p/TGFBR3 axis and the involvement of oxidative stress in this process. J Hazard Mater 406:124682. https://doi.org/10.1016/j.jhazmat.2020.124682
Roca-Agujetas V, de Dios C, Leston L, Mari M, Morales A, Colell A (2019) Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid Med Cell Longev 2019. doi:Artn 3809308 https://doi.org/10.1155/2019/3809308
Wang XY, Yang H, Wang MG, Yang DB, Wang ZY, Wang L (2017) Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis 8. doi:Artn E3099 https://doi.org/10.1038/Cddis.2017.475
Corsetti G, Romano C, Stacchiotti A, Pasini E, Dioguardi FS (2017) Endoplasmic reticulum stress and apoptosis triggered by sub-chronic lead exposure in mice spleen: a histopathological study. Biol Trace Elem Res 178(1):86–97. https://doi.org/10.1007/s12011-016-0912-z
Liu J, Dong C, Zhai Z, Tang L, Wang L (2021) Glyphosate-induced lipid metabolism disorder contributes to hepatotoxicity in juvenile common carp. Environ Pollut 269:116186. https://doi.org/10.1016/j.envpol.2020.116186
Ge Y, Huang M, Yao YM (2018) Autophagy and proinflammatory cytokines: interactions and clinical implications. Cytokine Growth Factor Rev 43:38–46. https://doi.org/10.1016/j.cytogfr.2018.07.001
Chen F, Bao HW, Xie HY, Tian G, Jiang TA (2019) Heat shock protein expression and autophagy after incomplete thermal ablation and their correlation. Int J Hyperth 36(1):95–103. https://doi.org/10.1080/02656736.2018.1536285
Jin X, Jia TT, Liu RH, Xu SW (2018) The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-gamma/PI3K/Akt pathway in chicken pancreas. J Hazard Mater 357:355–362. https://doi.org/10.1016/j.jhazmat.2018.06.003
Khalid A, Khudhair N, Huang H, Zheng P, Tian YG, Zhang GX (2016) Effects of dietary selenium supplementation on seminiferous tubules and SelW, GPx4, LHCGR, and ACE expression in chicken testis. Biol Trace Elem Res 173(1):202–209. https://doi.org/10.1007/s12011-016-0646-y
Huang H, Wang Y, An Y, Jiao WY, Xu YM, Han Q, Teng XJ, Teng XH (2019) Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology 131:146–152. https://doi.org/10.1016/j.theriogenology.2019.03.015
Huang H, Wang M, Hou L, Lin X, Pan S, Zheng P, Zhao Q (2021) A potential mechanism associated with lead-induced spermatogonia and Leydig cell toxicity and mitigative effect of selenium in chicken. Ecotoxicol Environ Saf 209:111671. https://doi.org/10.1016/j.ecoenv.2020.111671
Huang H, Li XY, Wang ZM, Lin X, Tian YG, Zhao Q, Zheng P (2020) Anti-inflammatory effect of selenium on lead-induced testicular inflammation by inhibiting NLRP3 inflammasome activation in chickens. Theriogenology 155:139–149. https://doi.org/10.1016/j.theriogenology.2020.06.015
Huang H, Chen JQ, Sun Q, Liu YH, Tang Y, Teng XH (2020) NLRP3 inflammasome is involved in the mechanism of mitigative effect of selenium on lead-induced inflammatory damage in chicken kidneys. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-020-11322-w
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90(5):1193–1209. https://doi.org/10.1007/s00204-015-1547-0
Leidens D, Bianchini A, Varela AS, Barcarolli IF, Rosa CE, Bonnel J, Calabuig CP, Corcini CD (2018) Effects of experimental lead exposure on testis of the chestnut capped blackbird Chrysomus ruficapillus. Bull Environ Contam Toxicol 100(3):324–330. https://doi.org/10.1007/s00128-017-2227-y
Asad A, Hamid S, Qamar K (2018) Effect of lead acetate on basement membrane of seminiferous tubules of adult rat testis and protective effects of Ficus carica: a histological study. J Coll Physicians Surg Pak 28(10):731–734
Reshma Anjum M, Sreenivasula Reddy P (2015) Recovery of lead-induced suppressed reproduction in male rats by testosterone. Andrologia 47(5):560–567. https://doi.org/10.1111/and.12303
Zheng SF, Wang SC, Zhang QJ, Zhang ZW, Xu SW (2020) Avermectin inhibits neutrophil extracellular traps release by activating PTEN demethylation to negatively regulate the PI3K-ERK pathway and reducing respiratory burst in carp. J Hazard Mater 389. doi:ARTN 121885 https://doi.org/10.1016/j.jhazmat.2019.121885
Wang LQ, Wang LX, Shi X, Xu SW (2020) Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J Hazard Mater 398. doi:ARTN 122905 https://doi.org/10.1016/j.jhazmat.2020.122905
Chen JQ, Xu YM, Han Q, Yao YC, Xing HJ, Teng XH (2019) Immunosuppression, oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): application of transcriptome analysis in risk assessment of environmental contaminant cadmium. J Hazard Mater 366:386–394. https://doi.org/10.1016/j.jhazmat.2018.12.014
Lopes ACBA, Peixe TS, Mesas AE, Paoliello MMB (2016) Lead exposure and oxidative stress: a systematic review. Rev Environ Contam Toxicol 236:193–238. https://doi.org/10.1007/978-3-319-20013-2_3
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128(4):501–523
Jiao X, Yang K, An Y, Teng X, Teng X (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res Int 24(8):7555–7564. https://doi.org/10.1007/s11356-016-8329-y
Kou H, Ya J, Gao X, Zhao H (2020) The effects of chronic lead exposure on the liver of female Japanese quail (Coturnix japonica): histopathological damages, oxidative stress and AMP-activated protein kinase based lipid metabolism disorder. Ecotoxicol Environ Saf 190:110055. https://doi.org/10.1016/j.ecoenv.2019.110055
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539. https://doi.org/10.2174/1568026013394831
Zhang Y, Liu Q, Yin H, Li S (2020) Cadmium exposure induces pyroptosis of lymphocytes in carp pronephros and spleens by activating NLRP3. Ecotoxicol Environ Saf 202:110903. https://doi.org/10.1016/j.ecoenv.2020.110903
Chen MH, Li XJ, Shi QX, Zhang ZW, Xu SW (2019) Hydrogen sulfide exposure triggers chicken trachea inflammatory injury through oxidative stress-mediated FOS/IL8 signaling. J Hazard Mater 368:243–254. https://doi.org/10.1016/j.jhazmat.2019.01.054
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer how are they linked? Free Radic Biol Med 49(11):1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Liu J, Wang S, Zhang Q, Li X, Xu S (2019) Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics : integrated biometal science 12:54–64. https://doi.org/10.1039/c9mt00216b
Abd El-Ghffar EA, Al-Sayed E, Shehata SM, Eldahshan OA, Efferth T (2018) The protective role of Ocimum basilicum L. (Basil) against aspirin-induced gastric ulcer in mice: impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct 9(8):4457–4468. https://doi.org/10.1039/c8fo00538a
Kalmar B, Greensmith L (2009) Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev 61(4):310–318. https://doi.org/10.1016/j.addr.2009.02.003
Xing M, Jin X, Wang J, Shi Q, Cai J, Xu S (2017) The antagonistic effect of selenium on lead-induced immune dysfunction via recovery of cytokine and heat shock protein expression in chicken neutrophils. Biol Trace Elem Res 185:162–169. https://doi.org/10.1007/s12011-017-1200-2
Huang H, Xiaoyan J, Yanmin X, Qi H, Wanying J, Yanyan L, Li S, Xiaohua T (2019) Dietary selenium supplementation alleviates immune toxicity in the hearts of chickens with lead-added drinking water. Avian Pathol:1–22. doi:https://doi.org/10.1080/03079457.2019.1572102
Li JF, Qi XW, Jiang BL, Huang T, Luo L, Liu SX, Yin ZM (2019) Phosphorylated heat shock protein 27 inhibits lipopolysaccharide-induced inflammation in Thp1 cells by promoting TLR4 endocytosis, ubiquitination, and degradation. Inflammation 42(5):1788–1799. https://doi.org/10.1007/s10753-019-01041-x
Sulistyowati E, Lee MY, Wu LC, Hsu JH, Dai ZK, Wu BN, Lin MC, Yeh JL (2018) Exogenous heat shock cognate protein 70 suppresses LPS-induced inflammation by down-regulating NF-kappaB through MAPK and MMP-2/-9 pathways in macrophages. Molecules 23(9). https://doi.org/10.3390/molecules23092124
Zhao Y, Li Z-F, Zhang D, Wang Z-Y, Wang L (2021) Quercetin alleviates cadmium-induced autophagy inhibition via TFEB-dependent lysosomal restoration in primary proximal tubular cells. Ecotoxicol Environ Saf 208:111743. https://doi.org/10.1016/j.ecoenv.2020.111743
Zou H, Sun J, Wu B, Yuan Y, Gu J, Bian J, Liu X, Liu Z (2020) Effects of cadmium and/or lead on autophagy and liver injury in rats. Biol Trace Elem Res 198:206–215. https://doi.org/10.1007/s12011-020-02045-7
Song XB, Liu G, Liu F, Yan ZG, Wang ZY, Liu ZP, Wang L (2017) Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis 8. doi:Artn E2863https://doi.org/10.1038/Cddis.2017.262
Han Y, Li C, Su M, Wang Z, Jiang N, D S (2017) Antagonistic effects of selenium on lead-induced autophagy by influencing mitochondrial dynamics in the spleen of chickens. Oncotarget 8 (20):33725–33735
Cuervo AM, Dice JF (1998) Lysosomes, a meeting point of proteins, chaperones, and proteases. J Mol Med 76(1):6–12. https://doi.org/10.1007/s001090050185
Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456(7219):264–268. https://doi.org/10.1038/nature07383
Yoshizaki T, Kusunoki C, Kondo M, Yasuda M, Kume S, Morino K, Sekine O, Ugi S, Uzu T, Nishio Y, Kashiwagi A, Maegawa H (2012) Autophagy regulates inflammation in adipocytes. Biochem Biophys Res Commun 417(1):352–357. https://doi.org/10.1016/j.bbrc.2011.11.114
Zhang YM, Liu QQ, Yin H, Min YH, Li S (2020) Selenium deficiency causes immune damage by activating the DUSP1/NF-kappa B pathway and endoplasmic reticulum stress in chicken spleen. Food Funct 11(7):6467–6475. https://doi.org/10.1039/d0fo00394h
Qin L, Zhang Y, Wan C, Wang Z, Cong Y, Li S (2020) MiR-196-5p involvement in selenium deficiency-induced immune damage via targeting of NFκBIA in the chicken trachea. Metallomics 12(11):1679–1692. https://doi.org/10.1039/d0mt00164c
Ozkan-Yilmaz F, Ozluer-Hunt A, Gunduz SG, Berkoz M, Yalin S (2014) Effects of dietary selenium of organic form against lead toxicity on the antioxidant system in Cyprinus carpio. Fish Physiol Biochem 40(2):355–363. https://doi.org/10.1007/s10695-013-9848-9
Wang Y, Wang KX, Huang H, Gu XH, Teng XH (2017) Alleviative effect of selenium on inflammatory damage caused by lead via inhibiting inflammatory factors and heat shock proteins in chicken testes. Environ Sci Pollut Res Int 24(15):13405–13413. https://doi.org/10.1007/s11356-017-8785-z
Liu R, Jia T, Cui Y, Lin H, Li S (2017) The protective effect of selenium on the chicken pancreas against cadmium toxicity via alleviating oxidative stress and autophagy. Biol Trace Elem Res 64:575–582. https://doi.org/10.1007/s12011-017-1186-9
Li M, Gao JQ, Li XW (2005) Antagonistic action of selenium against the toxicity of lead. Wei sheng yan jiu = Journal of hygiene research 34(3):375–377
Authorship Contribution Statement
Size Wang and Lulu Hou performed experiments and drafted the manuscript. Min Wang and Rui Feng helped to perform the experiments. Xu Lin contributed to the experiment preparation. Shifeng Pan and Qian Zhao made contribution to analyze the data and the technical assistance. He Huang was responsible for research design and revising the manuscript.
Data Availability Statement
All data in the current study are available from the corresponding author on reasonable request.
Funding
The study was supported by the Heilongjiang Province on Natural Fund Project of China (no. LH2019C026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures used in this experiment were approved by the Northeast Agricultural University’s Institutional Animal Care and Use Committee under the approved protocol number SRM-06. Date of approval: 1 January 2018.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Hou, L., Wang, M. et al. Selenium-Alleviated Testicular Toxicity by Modulating Inflammation, Heat Shock Response, and Autophagy Under Oxidative Stress in Lead-Treated Chickens. Biol Trace Elem Res 199, 4700–4712 (2021). https://doi.org/10.1007/s12011-021-02588-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02588-3